Abstract
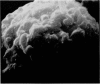
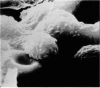
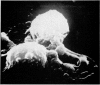
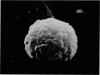
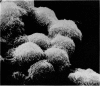
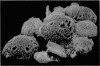
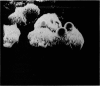
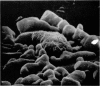
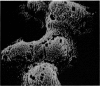
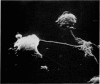
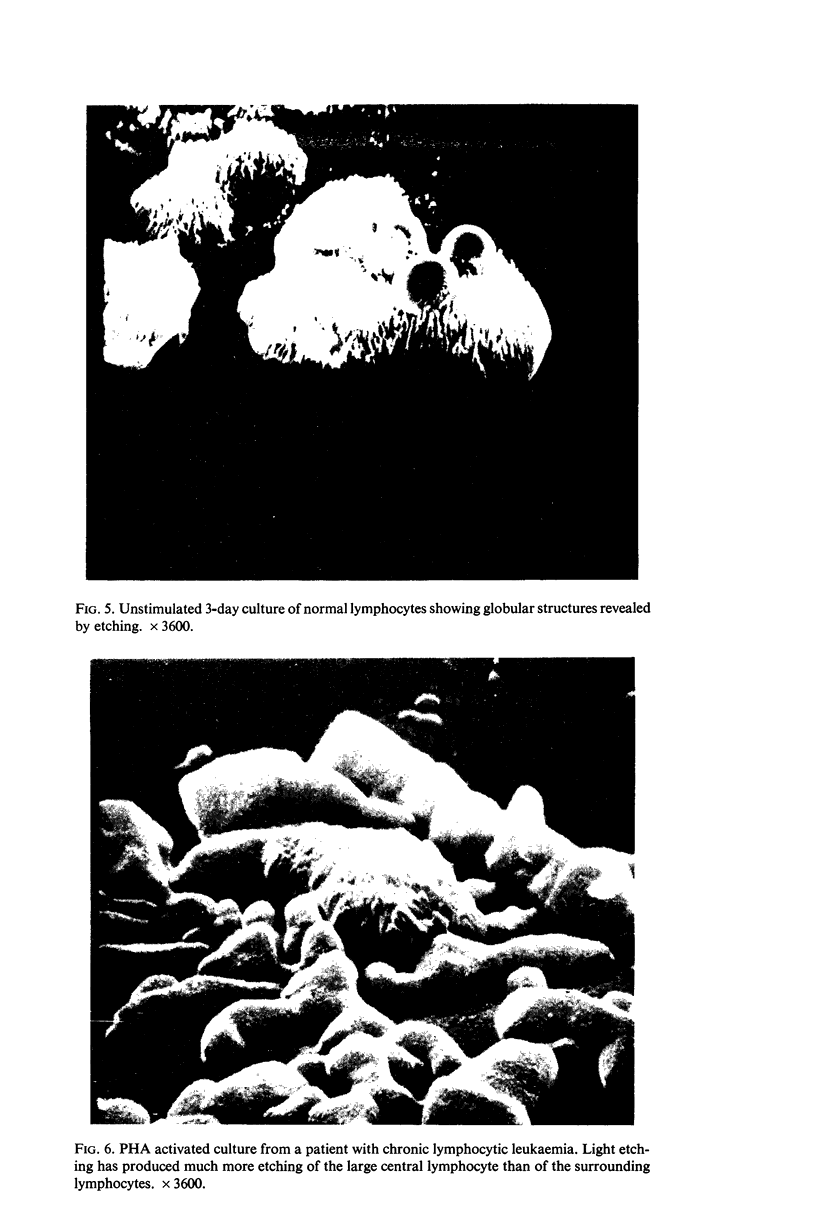
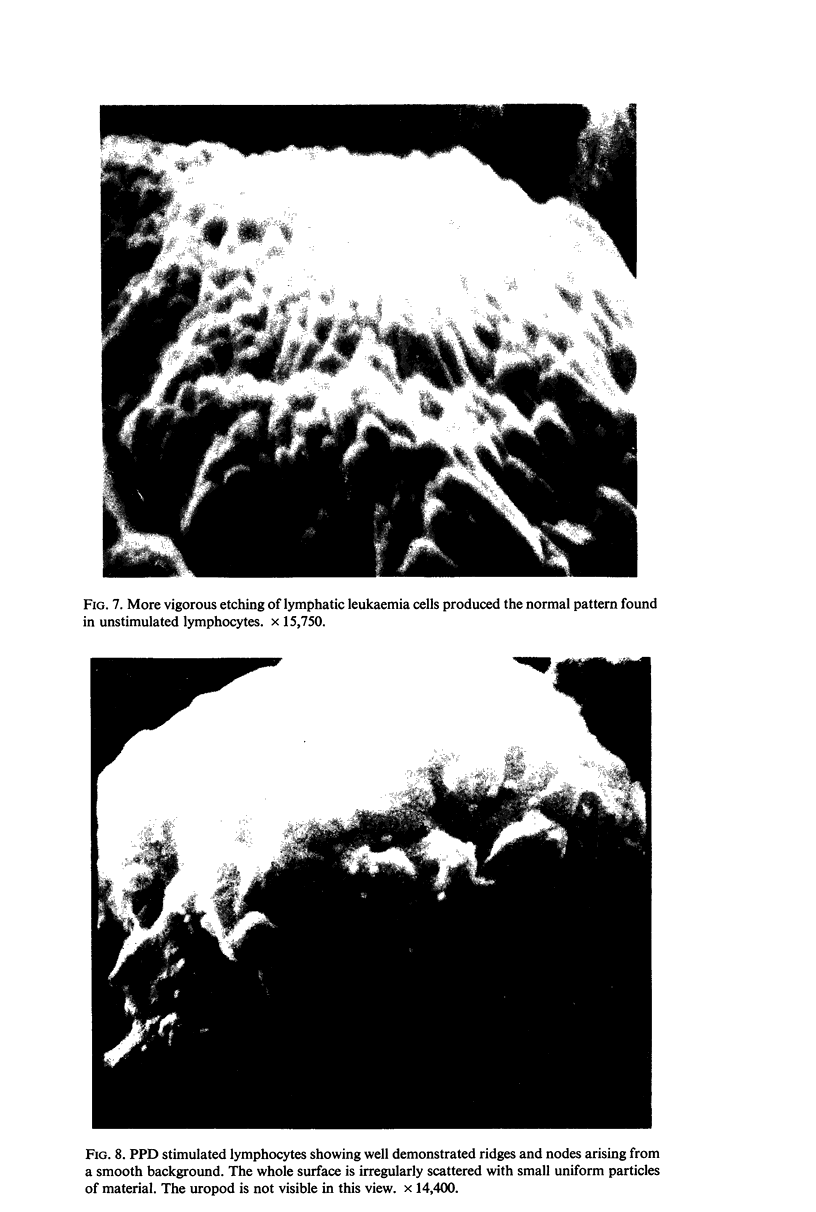
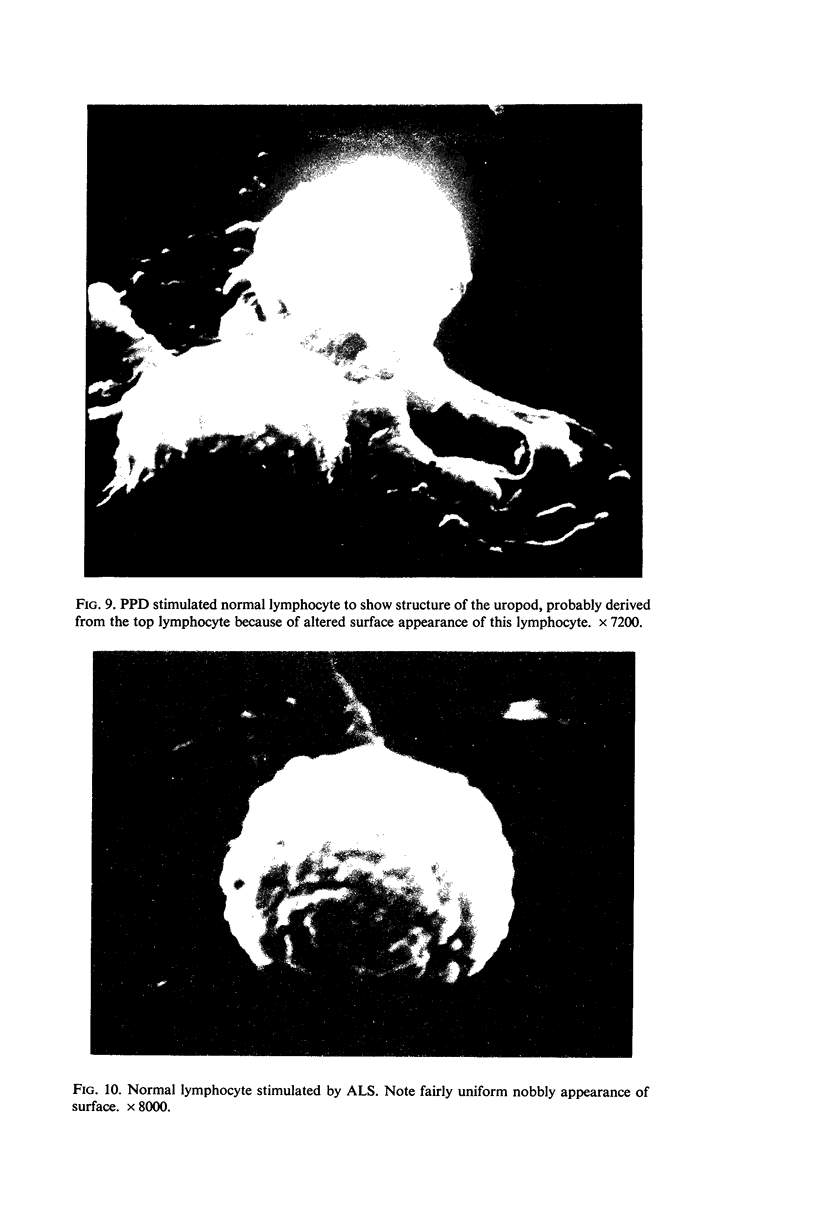
Scanning electron microscopy has been used to examine the surface structure of normal and leukaemic lymphocytes and the effect of specific and non-specific mitogens on them. Both types of mitogens produce an increase in the surface irregularity of the normal cell but not of the leukaemic cell. The uropod is seen to consist of a fork-like structure the prongs of which are joined together by a fine membrane.
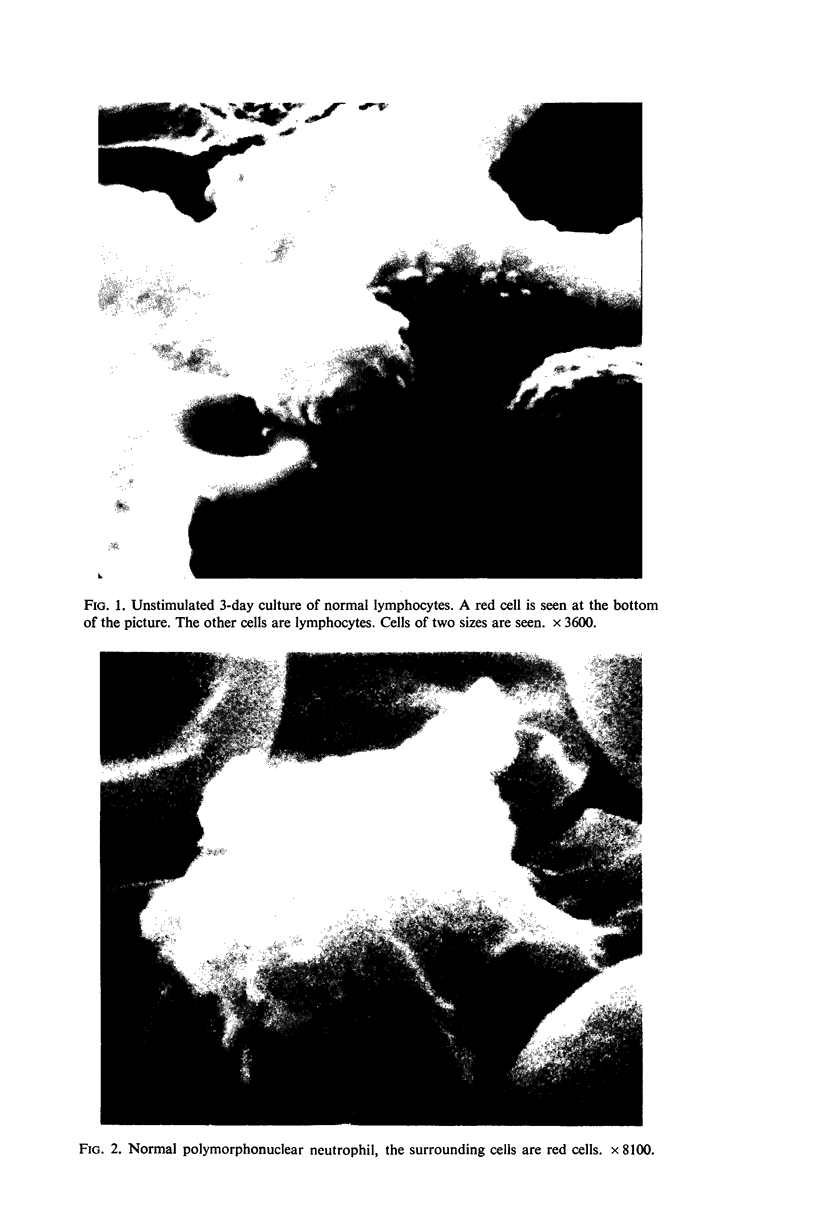
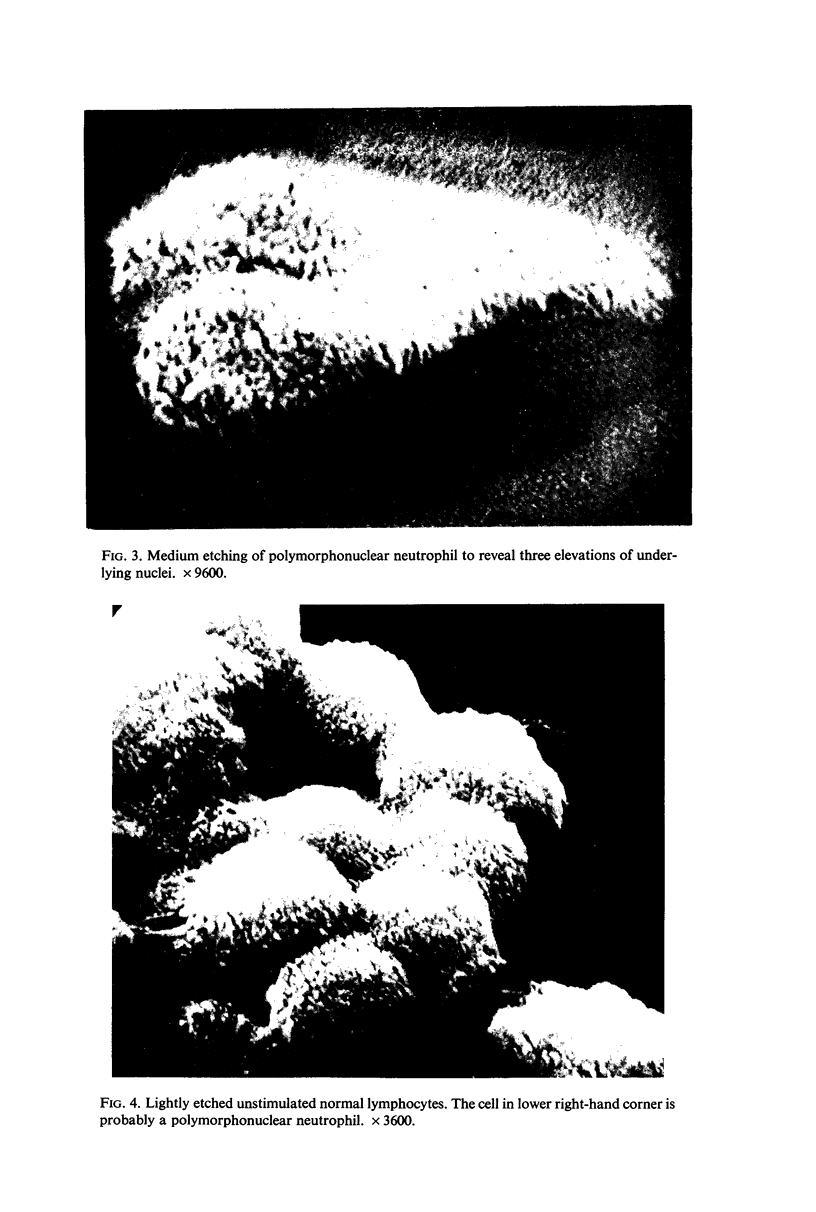
The use of the technique of ionic etching on the surface of the lymphocyte has shown variation in the physical hardness of the surface of the lymphocyte as a result of non-specific mitogen stimulation only. The surface of the leukaemic lymphocyte was considerably more resistant to etching than normal.
These results in conjunction with the results of other workers suggest that non-specific activation picks out the thymic dependent cells because these have weaker cell surface membranes and by corollary the difficulty in stimulating leukaemic lymphocytes is due to the increased resilience of this surface membrane. Thus the differentiation of thymic dependent lymphocytes by non-specific stimulation may be purely fortuitous and not directly related to their physiological function.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biberfeld P. Uropod formation in phytohaemagglutinin (PHA) stimulated lymphocytes. Exp Cell Res. 1971 Jun;66(2):433–445. doi: 10.1016/0014-4827(71)90698-7. [DOI] [PubMed] [Google Scholar]
- Bloch-Shtacher N., Hirschhorn K., Uhr J. W. The response of lymphocytes from non-immunized humans to antigen-antibody complexes. Clin Exp Immunol. 1968 Nov;3(9):889–899. [PMC free article] [PubMed] [Google Scholar]
- COULSON A. S., CHALMERS D. G. SEPARATION OF VIABLE LYMPHOCYTES FROM HUMAN BLOOD. Lancet. 1964 Feb 29;1(7331):468–469. doi: 10.1016/s0140-6736(64)90799-8. [DOI] [PubMed] [Google Scholar]
- Carr I., Clarke J. A., Salsbury A. J. The surface structure of mouse peritoneal cells--a study with the scanning electron microscope. J Microsc. 1969;89(1):105–111. doi: 10.1111/j.1365-2818.1969.tb00654.x. [DOI] [PubMed] [Google Scholar]
- Clarke J. A., Salsbury A. J., Rowland G. F. Surface ultrastructure of human leucocytes, mouse macrophages and rat liver cells, and of isolated nuclei and nucleoli. Br J Haematol. 1968 May;14(5):533–542. doi: 10.1111/j.1365-2141.1968.tb07005.x. [DOI] [PubMed] [Google Scholar]
- Clarke J. A., Salsbury A. J., Willoughby D. A. The effects of various antisera on the surfaces of sensitised rat lymph-node cells. J Pathol Bacteriol. 1968 Jul;96(1):235–238. doi: 10.1002/path.1700960129. [DOI] [PubMed] [Google Scholar]
- Conard R. A., Demoise C. F. Preparation and purification of tritiated phytohemagglutinin and studies of cellular localization in human leukocyte cultures. Blood. 1970 Jan;35(1):44–55. [PubMed] [Google Scholar]
- Cooper H. L., Rubin A. D. Lymphocyte RNA metabolism. Comparison between antigen and phytohaemagglutinin stimulation. Lancet. 1965 Oct 9;2(7415):723–724. doi: 10.1016/s0140-6736(65)90460-5. [DOI] [PubMed] [Google Scholar]
- Folb P. I., Trounce J. R. Selective pharmacological inhibition of lymphocyte stimulation. Lancet. 1971 Jul 24;2(7717):221–222. doi: 10.1016/s0140-6736(71)90941-x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Torrigiani G., Roitt I. M. Inhibition of human mixed lymphocyte reaction by antibodies to immunoglobulin light chain determinants. Clin Exp Immunol. 1971 Sep;9(3):313–328. [PMC free article] [PubMed] [Google Scholar]
- Hersh E. M., Harris J. E. Macrophage-lymphocyte interaction in the antigen-induced blastogenic response of human peripheral blood leukocytes. J Immunol. 1968 Jun;100(6):1184–1194. [PubMed] [Google Scholar]
- Holt L. J., Ling N. R., Stanworth D. R. The effect of heterologous antisera and rheumatoid factor on the synthesis of DNA and protein by human peripheral lymphocytes. Immunochemistry. 1966 Sep;3(5):359–371. doi: 10.1016/0019-2791(66)90174-1. [DOI] [PubMed] [Google Scholar]
- Killander D., Rigler R. Activation of deoxyribonucleoprotein in human leucocytes stimulated by phytohemagglutinin. I. Kinetics of the binding of acridine orange to deoxyribonucleoprotein. Exp Cell Res. 1969 Feb;54(2):163–170. doi: 10.1016/0014-4827(69)90228-6. [DOI] [PubMed] [Google Scholar]
- Klein E., Eskeland T., Inoue M., Strom R., Johansson B. Surface immunoglobulin-moieties on lymphoid cells. Exp Cell Res. 1970 Sep;62(1):133–148. doi: 10.1016/0014-4827(79)90515-9. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- Lewis S. M., Osborn J. S., Stuart P. R. Demonstration of an internal structure within the red blood cell by ion etching and scanning electronmicroscopy. Nature. 1968 Nov 9;220(5167):614–616. doi: 10.1038/220614a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. M., Stuart P. R. Ultrastructure of the red blood cell. Proc R Soc Med. 1970 May;63(5):465–468. [PMC free article] [PubMed] [Google Scholar]
- Lindahl-Kiessling K., Mattsson A. Mechanism of phytohemagglutinin (PHA) action. IV. Effect of some metabolic inhibitors on binding of PHA to lymphocytes and the stimulatory potential of PHA-pretreated cells. Exp Cell Res. 1971 Apr;65(2):307–312. doi: 10.1016/0014-4827(71)90006-1. [DOI] [PubMed] [Google Scholar]
- Marshall W. H., Roberts K. B. Continuous cinematography of human lymphocytes cultured with a phytohaemagglutinin including observations on cell division and interphase. Q J Exp Physiol Cogn Med Sci. 1965 Oct;50(4):361–374. doi: 10.1113/expphysiol.1965.sp001803. [DOI] [PubMed] [Google Scholar]
- Mazzei D., Novi C., Bazzi C. Mitogenic action of papain. Lancet. 1966 Oct 8;2(7467):802–803. doi: 10.1016/s0140-6736(66)90406-5. [DOI] [PubMed] [Google Scholar]
- McDonald L. W., Hayes T. L. Correlation of scanning electron microscope and light microscope images of individual cells in human blood and blood clots. Exp Mol Pathol. 1969 Apr;10(2):186–198. doi: 10.1016/0014-4800(69)90039-2. [DOI] [PubMed] [Google Scholar]
- McFarland W., Heilman D. H., Moorhead J. F. Functional anatomy of the lymphocyte in immunological reactions in vitro. J Exp Med. 1966 Nov 1;124(5):851–858. doi: 10.1084/jem.124.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarland W. Microspikes on the lymphocyte uropod. Science. 1969 Feb 21;163(3869):818–820. doi: 10.1126/science.163.3869.818. [DOI] [PubMed] [Google Scholar]
- McFarland W., Schechter G. P. The lymphocyte in immunological reactions in vitro: ultrastructural studies. Blood. 1970 May;35(5):683–688. [PubMed] [Google Scholar]
- Michaelis T. W., Larrimer N. R., Metz E. N., Balcerzak S. P. Surface morphology of human leukocytes. Blood. 1971 Jan;37(1):23–30. [PubMed] [Google Scholar]
- Oppenheim J. J. Immunological relevance of antigen and antigen antibody complex induced lymphocyte transformation. Ann Allergy. 1969 Jul;27(7):305–315. [PubMed] [Google Scholar]
- Pogo B. G., Allfrey V. G., Mirsky A. E. RNA synthesis and histone acetylation during the course of gene activation in lymphocytes. Proc Natl Acad Sci U S A. 1966 Apr;55(4):805–812. doi: 10.1073/pnas.55.4.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin A. D., Havemann K., Dameshek W. Studies in chronic lymphocytic leukemia: further studies of the proliferative abnormality of the blood lymphocyte. Blood. 1969 Feb;33(2):313–328. [PubMed] [Google Scholar]
- Salsbury A. J., Clarke J. A. New method for detecting changes in the surface appearance of human red blood cells. J Clin Pathol. 1967 Jul;20(4):603–610. doi: 10.1136/jcp.20.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonson L. G., Shklair I. L. Scanning electron microscopy of the antigen-antibody complex. Experientia. 1970 Nov 15;26(11):1287–1288. doi: 10.1007/BF01898024. [DOI] [PubMed] [Google Scholar]
- Smithwick E. M., Berkovich S. In vitro suppression of the lymphocyte response to tuberculin by live measles virus. Proc Soc Exp Biol Med. 1966 Oct;123(1):276–278. doi: 10.3181/00379727-123-31465. [DOI] [PubMed] [Google Scholar]
- Stodolnik-Barańska W. Lymphoblastoid transformation of lymphocytes in vitro after microwave irradiation. Nature. 1967 Apr 1;214(5083):102–103. doi: 10.1038/214102a0. [DOI] [PubMed] [Google Scholar]
- Thomson A. E., Bull J. M., Robinson M. A. A procedure for separating viable lymphocytes from human blood and some studies on their susceptibility to hypotonic shocks. Br J Haematol. 1966 Jul;12(4):433–446. doi: 10.1111/j.1365-2141.1966.tb05652.x. [DOI] [PubMed] [Google Scholar]
- Thomson A. E., Robinson M. A., Wetherley-Mein G. Heterogeneity of lymphocytes in chronic lymphocytic leukaemia. Lancet. 1966 Jul 23;2(7456):200–202. doi: 10.1016/s0140-6736(66)92480-9. [DOI] [PubMed] [Google Scholar]
- Turk A., Glade P. R., Chessin L. N. Blast-like transformation induced in peripheral blood lymphocytes by cellular injury: a comparison of sonication and phytohemagglutinin. Blood. 1969 Feb;33(2):329–340. [PubMed] [Google Scholar]
- Tursi A., Greaves M. F., Torrigiani G., Playfair J. H., Roitt I. M. Response to phytohaemagglutinin of lymphocytes from mice treated with anti-lymphocyte globulin. Immunology. 1969 Nov;17(5):801–811. [PMC free article] [PubMed] [Google Scholar]
- VASSAR P. S., CULLIN C. F. CELL SURFACE EFFECTS OF PHYTOHAEMAGGLUTININ. Nature. 1964 May 9;202:610–611. doi: 10.1038/202610a0. [DOI] [PubMed] [Google Scholar]
- Weiss L. Some comments on RNA as a component of the cell periphery. Wistar Inst Symp Monogr. 1968;8:73–89. [PubMed] [Google Scholar]
- Zucker-Franklin D. The ultrastructure of lymphocytes. Semin Hematol. 1969 Jan;6(1):4–27. [PubMed] [Google Scholar]