Abstract
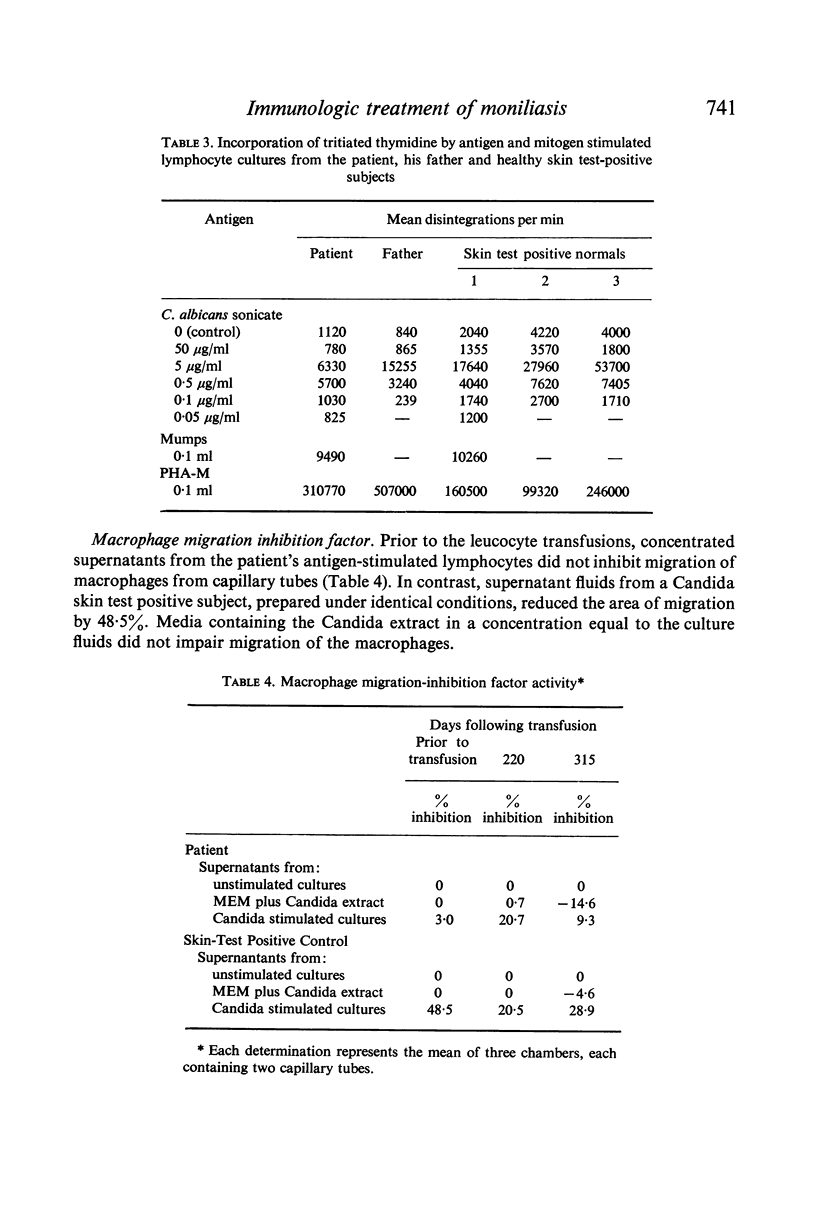
The immunological defect in a patient with chronic mucocutaneous moniliasis was characterized. While his Candida skin test was negative. exposure of his lymphocytes to candida extracts in vitro produced an increase in thymidine incorporation. Supernatants from cultures of antigen-stimulated lymphocytes did not contain macrophage migration-inhibition factor (MIF) activity.
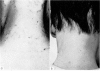
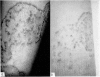
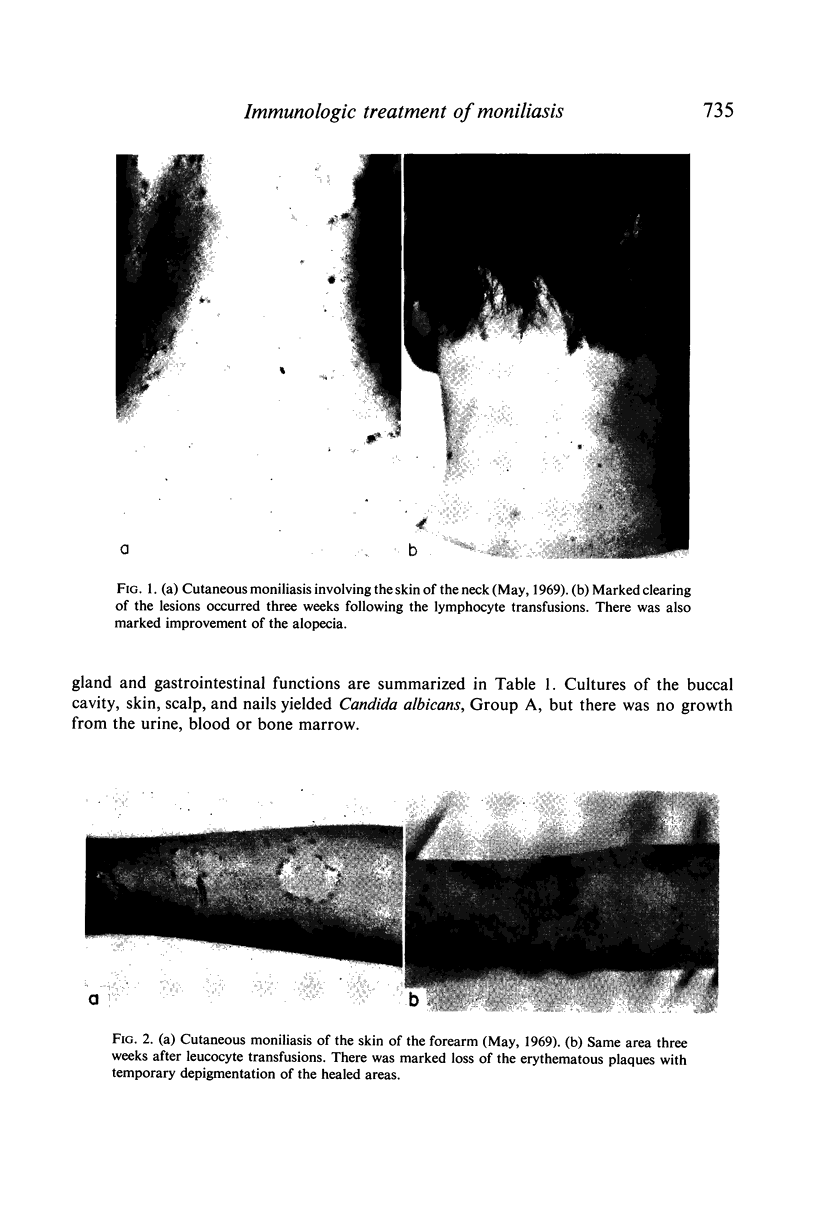
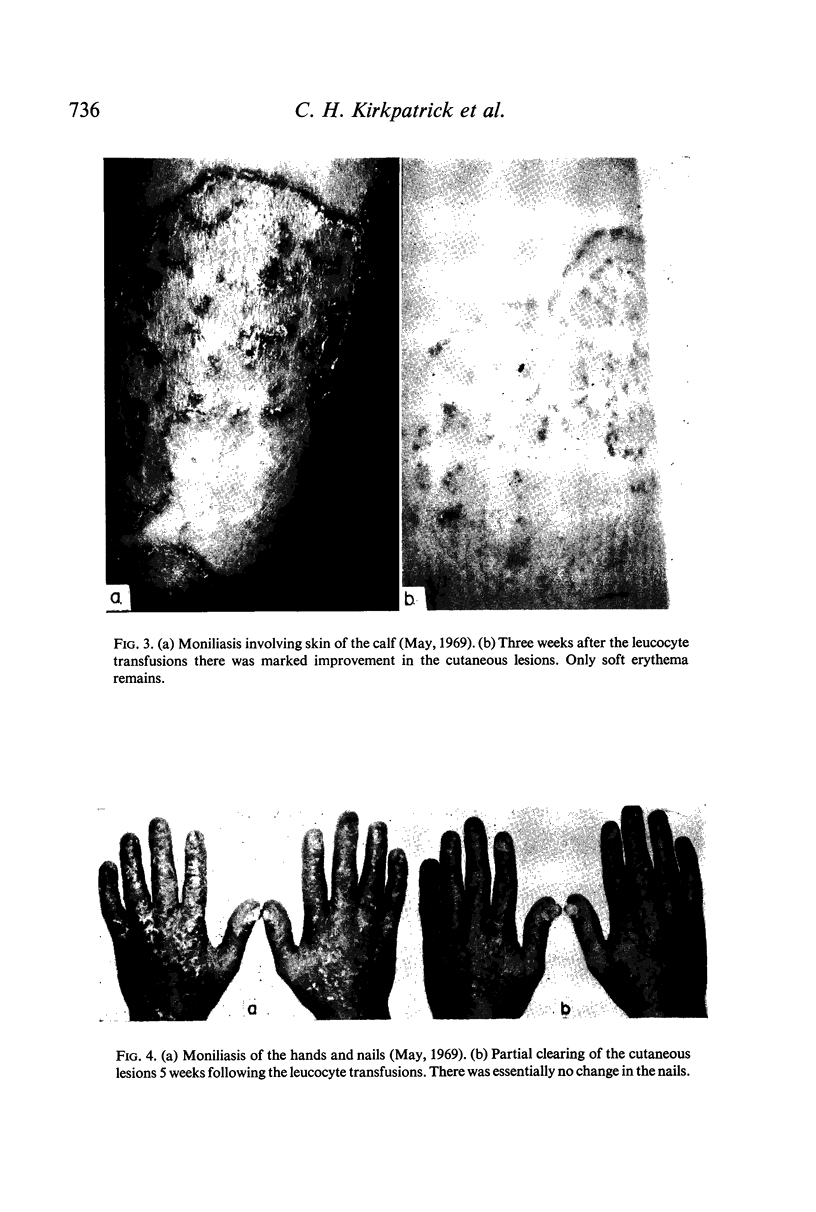
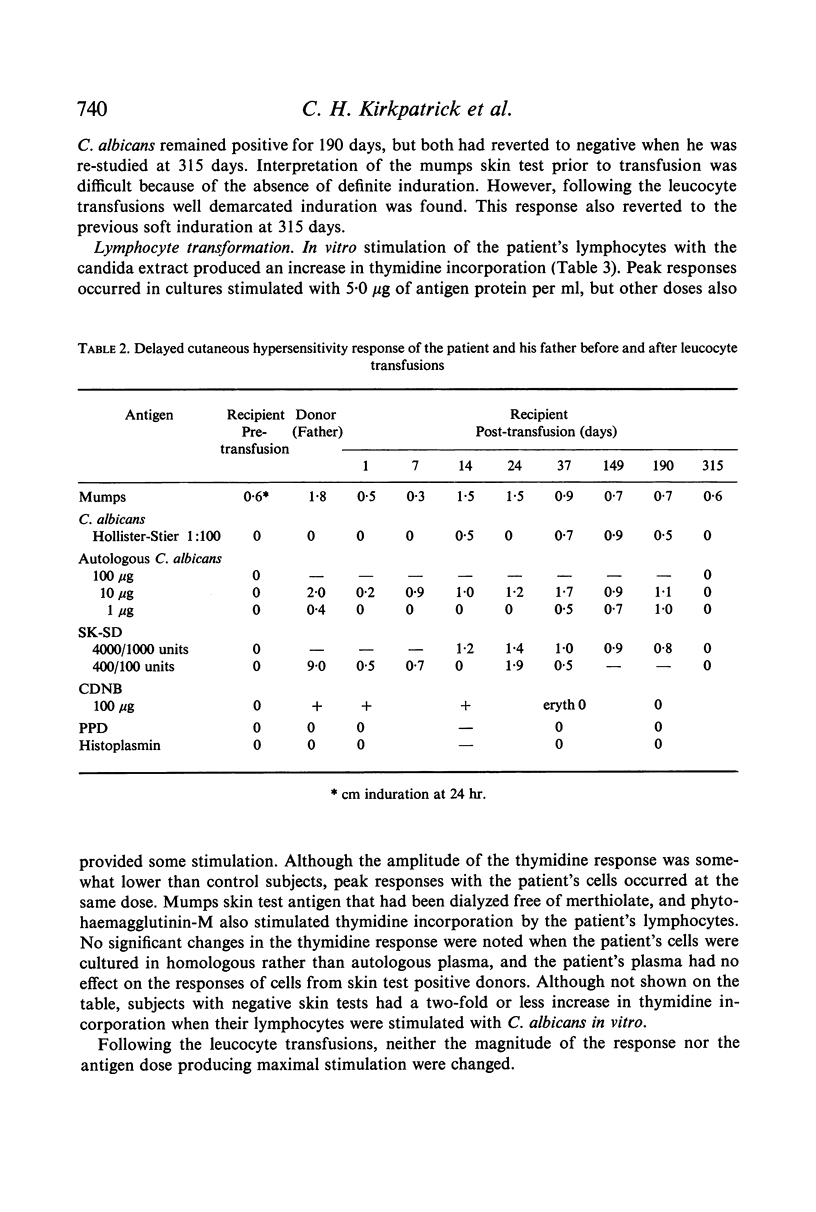
Restoration of the immune system with transfusions of immuno-competent allogeneic lymphocytes was accompanied by conversion of the Candida skin test to positive, and MIF production by his lymphocytes. During the period that his immune system remained intact, there was marked clearing of the moniliasis. Eight months following the transfusions, the moniliasis recurred and when restudied, the patient again had negative skin tests and insignificant MIF production.
These observations demonstrate the importance of mediators in the expression of delayed hypersensitivity and provide evidence of a role of cellular immunity in resistance to certain chronic fungal infections.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach F. H., Voynow N. K. One-way stimulation in mixed leukocyte cultures. Science. 1966 Jul 29;153(3735):545–547. doi: 10.1126/science.153.3735.545. [DOI] [PubMed] [Google Scholar]
- Bennett B., Bloom B. R. Reactions in vivo and in vitro produced by a soluble substance associated with delayed-type hypersensitivity. Proc Natl Acad Sci U S A. 1968 Mar;59(3):756–762. doi: 10.1073/pnas.59.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
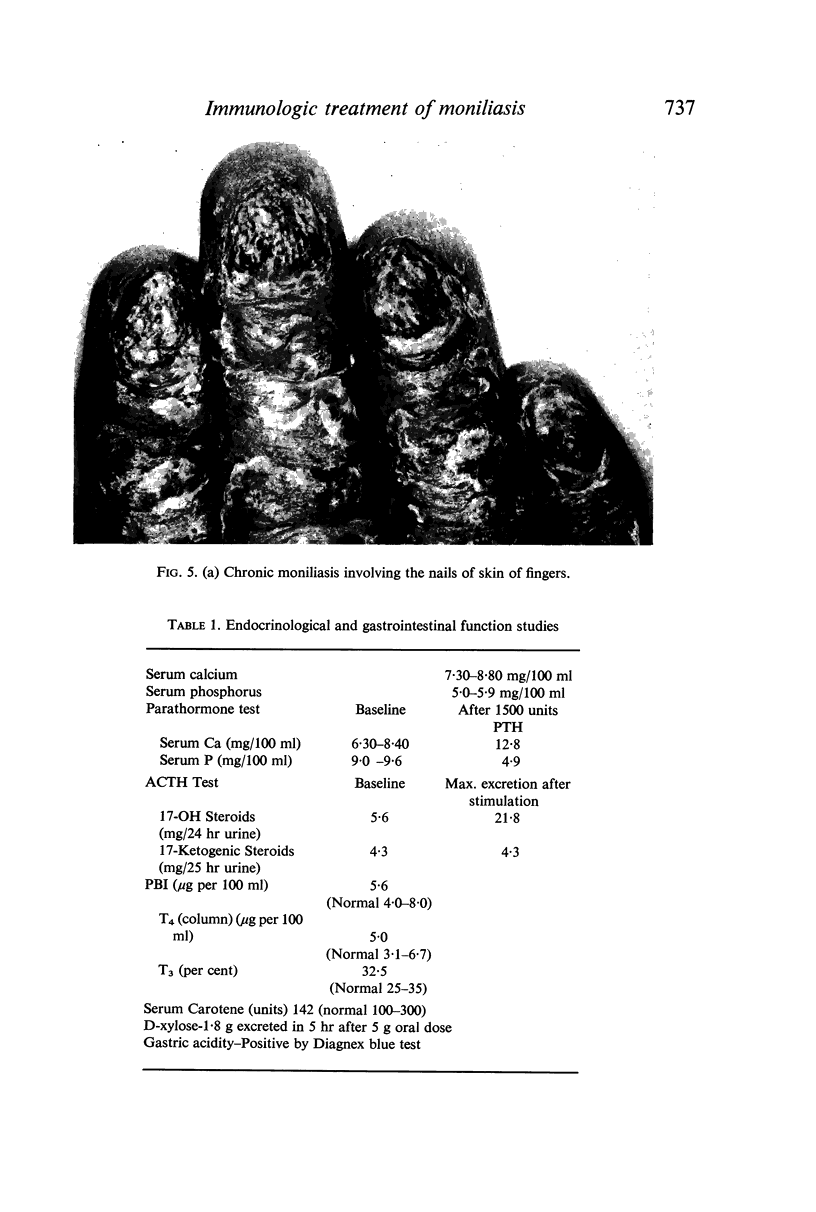
- Blizzard R. M., Gibbs J. H. Candidiasis: studies pertaining to its association with endocrinopathies and pernicious anemia. Pediatrics. 1968 Aug;42(2):231–237. [PubMed] [Google Scholar]
- Buckner D., Graw R. G., Jr, Eisel R. J., Henderson E. S., Perry S. Leukapheresis by continuous flow centrifugation (CFC) in patients with chronic myelocytic leukemia (CML). Blood. 1969 Feb;33(2):353–369. [PubMed] [Google Scholar]
- Cantor H., Asofsky R. Synergy among lymphoid cells mediating the graft-versus-host response. II. Synergy in graft-versus-host reactions produced by Balb-c lymphoid cells of differing anatomic origin. J Exp Med. 1970 Feb;131(2):235–246. doi: 10.1084/jem.131.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilgren R. A., Meuwissen H. J., Quie P. G., Good R. A., Hong R. The cellular immune defect in chronic mucocutaneous candidiasis. Lancet. 1969 Jun 28;1(7609):1286–1288. doi: 10.1016/s0140-6736(69)92223-5. [DOI] [PubMed] [Google Scholar]
- Chilgren R. A., Quie P. G., Meuwissen H. J., Hong R. Chronic mucocutaneous candidiasis, deficiency of delayed hypersensitivity, and selective local antibody defect. Lancet. 1967 Sep 30;2(7518):688–693. doi: 10.1016/s0140-6736(67)90974-9. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Chaperon E. A. Immunologic complementation between thymus and marrow cells--a model for the two-cell theory of immunocompetence. Transplant Rev. 1969;1:92–113. doi: 10.1111/j.1600-065x.1969.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Cleveland W. W., Fogel B. J., Brown W. T., Kay H. E. Foetal thymic transplant in a case of Digeorge's syndrome. Lancet. 1968 Dec 7;2(7580):1211–1214. doi: 10.1016/s0140-6736(68)91694-2. [DOI] [PubMed] [Google Scholar]
- Cohen R., Roth F. J., Delgado E., Ahearn D. G., Kalser M. H. Fungal flora of the normal human small and large intestine. N Engl J Med. 1969 Mar 20;280(12):638–641. doi: 10.1056/NEJM196903202801204. [DOI] [PubMed] [Google Scholar]
- David J. R. Macrophage migration. Fed Proc. 1968 Jan-Feb;27(1):6–12. [PubMed] [Google Scholar]
- Granger G. A., Williams T. W. Lymphocyte cytotoxicity in vitro: activation and release of a cytotoxic factor. Nature. 1968 Jun 29;218(5148):1253–1254. doi: 10.1038/2181253a0. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Roitt I. M., Rose M. E. Effect of bursectomy and thymectomy on the responses of chicken peripheral blood lymphocytes to phytohaemagglutinin. Nature. 1968 Oct 19;220(5164):293–295. doi: 10.1038/220293a0. [DOI] [PubMed] [Google Scholar]
- HUTER R. V., COLLINS H. S. The occurrence of opportunistic fungus infections in a cancer hospital. Lab Invest. 1962 Nov;11:1035–1045. [PubMed] [Google Scholar]
- Hermans P. E., Ulrich J. A., Markowitz H. Chronic mucocutaneous candidiasis as a surface expression of deep-seated abnormalities. Report of a syndrome of superficial candidiasis, absence of delayed hypersensitivity and aminoaciduria. Am J Med. 1969 Oct;47(4):503–519. doi: 10.1016/0002-9343(69)90181-8. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Chandler J. W., Schimke R. N. Chronic mucocutaneous moniliasis with impaired delayed hypersensitivity. Clin Exp Immunol. 1970 Mar;6(3):375–385. [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Cline M. J. Leukocyte myeloperoxidase deficiency and disseminated candidiasis: the role of myeloperoxidase in resistance to Candida infection. J Clin Invest. 1969 Aug;48(8):1478–1488. doi: 10.1172/JCI106114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louria D. B., Shannon D., Johnson G., Caroline L., Okas A., Taschdjian C. The susceptibility to moniliasis in children with endocrine hypofunction. Trans Assoc Am Physicians. 1967;80:236–249. [PubMed] [Google Scholar]
- Maini R. N., Bryceson A. D., Wolstencroft R. A., Dumonde D. C. Lymphocyte mitogenic factor in man. Nature. 1969 Oct 4;224(5214):43–44. doi: 10.1038/224043a0. [DOI] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Marmor M. F., Barnett E. V. Cutaneous anergy without systemic disease. A syndrome associated with mucocutaneous fungal infection. Am J Med. 1968 Jun;44(6):979–989. doi: 10.1016/0002-9343(68)90097-1. [DOI] [PubMed] [Google Scholar]
- Mickenberg I. D., Root R. K., Wolff S. M. Leukocytic function in hypogammaglobulinemia. J Clin Invest. 1970 Aug;49(8):1528–1538. doi: 10.1172/JCI106370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills J. A. The immunologic significance of antigen induced lymphocyte transformation in vitro. J Immunol. 1966 Aug;97(2):239–247. [PubMed] [Google Scholar]
- Mittal K. K., Mickey M. R., Singal D. P., Terasaki P. I. Serotyping for homotransplantation. 18. Refinement of microdroplet lymphocyte cytotoxicity test. Transplantation. 1968 Nov;6(8):913–927. doi: 10.1097/00007890-196811000-00006. [DOI] [PubMed] [Google Scholar]
- Mosier D. E., Coppleson L. W. A THREE-CELL INTERACTION REQUIRED FOR THE INDUCTION OF THE PRIMARY IMMUNE RESPONSE in vitro. Proc Natl Acad Sci U S A. 1968 Oct;61(2):542–547. doi: 10.1073/pnas.61.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry W. M., Jr, Chandler J. W., Jr, Chin T. D., Kirkpatrick C. H. Immunology of the mycoses. I. Depressed lymphocyte transformation in chronic histoplasmosis. J Immunol. 1968 Feb;100(2):436–443. [PubMed] [Google Scholar]
- REBUCK J. W., CROWLEY J. H. A method of studying leukocytic functions in vivo. Ann N Y Acad Sci. 1955 Mar 24;59(5):757–805. doi: 10.1111/j.1749-6632.1955.tb45983.x. [DOI] [PubMed] [Google Scholar]
- Rifkind D., Marchioro T. L., Schneck S. A., Hill R. B., Jr Systemic fungal infections complicating renal transplantation and immunosuppressive therapy. Clinical, microbiologic, neurologic and pathologic features. Am J Med. 1967 Jul;43(1):28–38. doi: 10.1016/0002-9343(67)90146-5. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Chilgren R. A., Hong R., David J. R. Transfer of cellular hypersensitivity in chronic mucocutaneous candidiasis monitored in vivo and in vitro. Cell Immunol. 1970 Sep;1(3):290–299. doi: 10.1016/0008-8749(70)90050-x. [DOI] [PubMed] [Google Scholar]
- Rocklin R. E., Meyers O. L., David J. R. An in vitro assay for cellular hypersensitivity in man. J Immunol. 1970 Jan;104(1):95–102. [PubMed] [Google Scholar]
- Sjöberg K. H. Moniliasis--an internal disease? Three cases of idiopathic hypoparathyroidism with moniliasis, steatorrhea, primary amenorrhea and pernicious anemia. Acta Med Scand. 1966 Feb;179(2):157–166. [PubMed] [Google Scholar]
- Thor D. E., Jureziz R. E., Veach S. R., Miller E., Dray S. Cell migration inhibition factor released by antigen from human peripheral lymphocytes. Nature. 1968 Aug 17;219(5155):755–757. doi: 10.1038/219755a0. [DOI] [PubMed] [Google Scholar]
- Valdimarsson H., Holt L., Riches H. R., Hobbs J. R. Lymphocyte abnormality in chronic mucocutaneous candidiasis. Lancet. 1970 Jun 13;1(7659):1259–1261. doi: 10.1016/s0140-6736(70)91742-3. [DOI] [PubMed] [Google Scholar]
- Ward P. A., Remold H. G., David J. R. Leukotactic factor produced by sensitized lymphocytes. Science. 1969 Mar 7;163(3871):1079–1081. doi: 10.1126/science.163.3871.1079. [DOI] [PubMed] [Google Scholar]