Abstract
The cytotoxic titre−1 of rabbit-anti-mouse thymocyte IgG (ALG), determined by a modified test in which the cells were first incubated with ALG, then washed and re-incubated with guinea-pig C, ranged from 3110 to 5470. The cytolytic efficiency (i.e. the reciprocal of the mean number of IgG molecules attached per cell under conditions in which 50% lysis occurs in the presence of C), measured with 131I-ALG, was about 0·002%. When γ2 IgG from guinea-pigs immunized with rabbit IgG was added prior to the second incubation the cytotoxic titre was augmented up to ten-fold and the cytotoxic efficiency up to twenty-fold. The same γ2 preparation resulted in up to 300-fold augmentation in a haemolytic system. In the thymocyte-ALG system, unlike the haemolytic system, augmentation was maximal at reduced concentrations of γ2. The mechanism of augmentation by γ2 has been investigated using 131I-ALG and 125I- γ2 IgG.
Full text
PDF




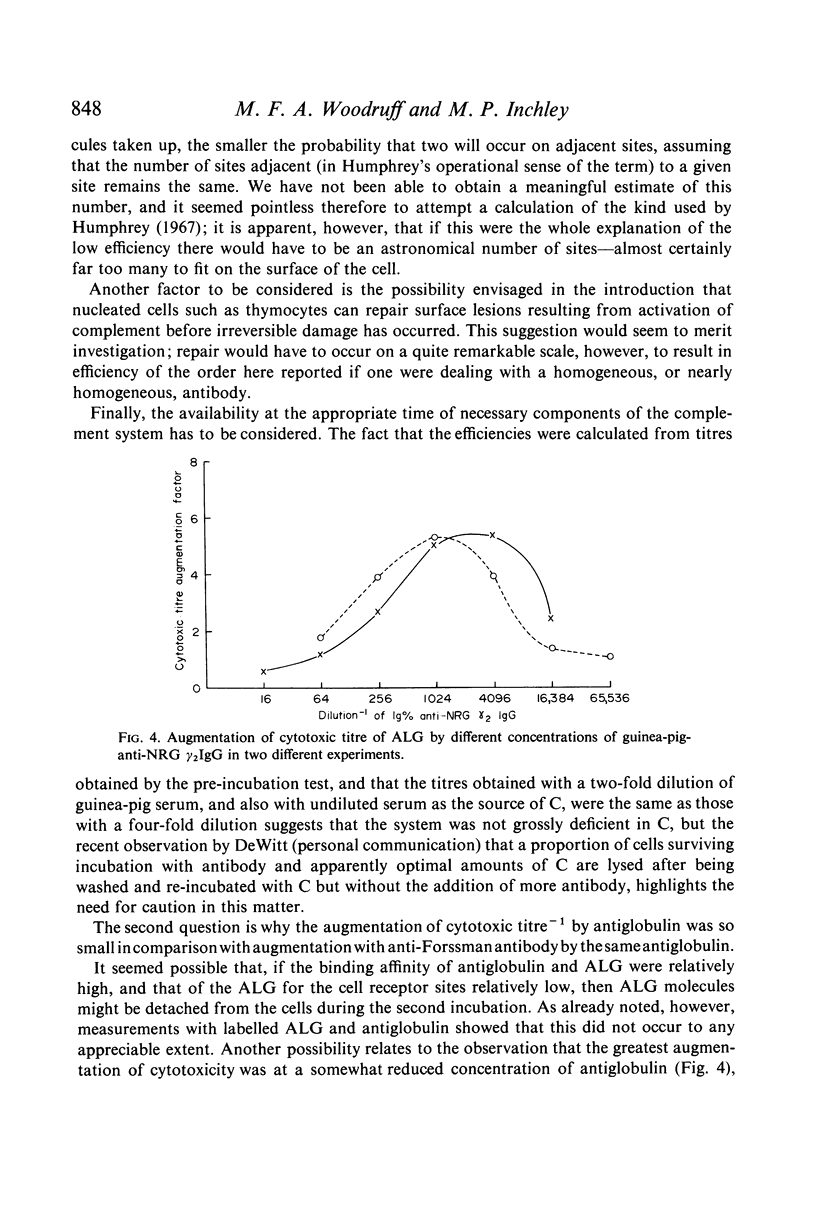
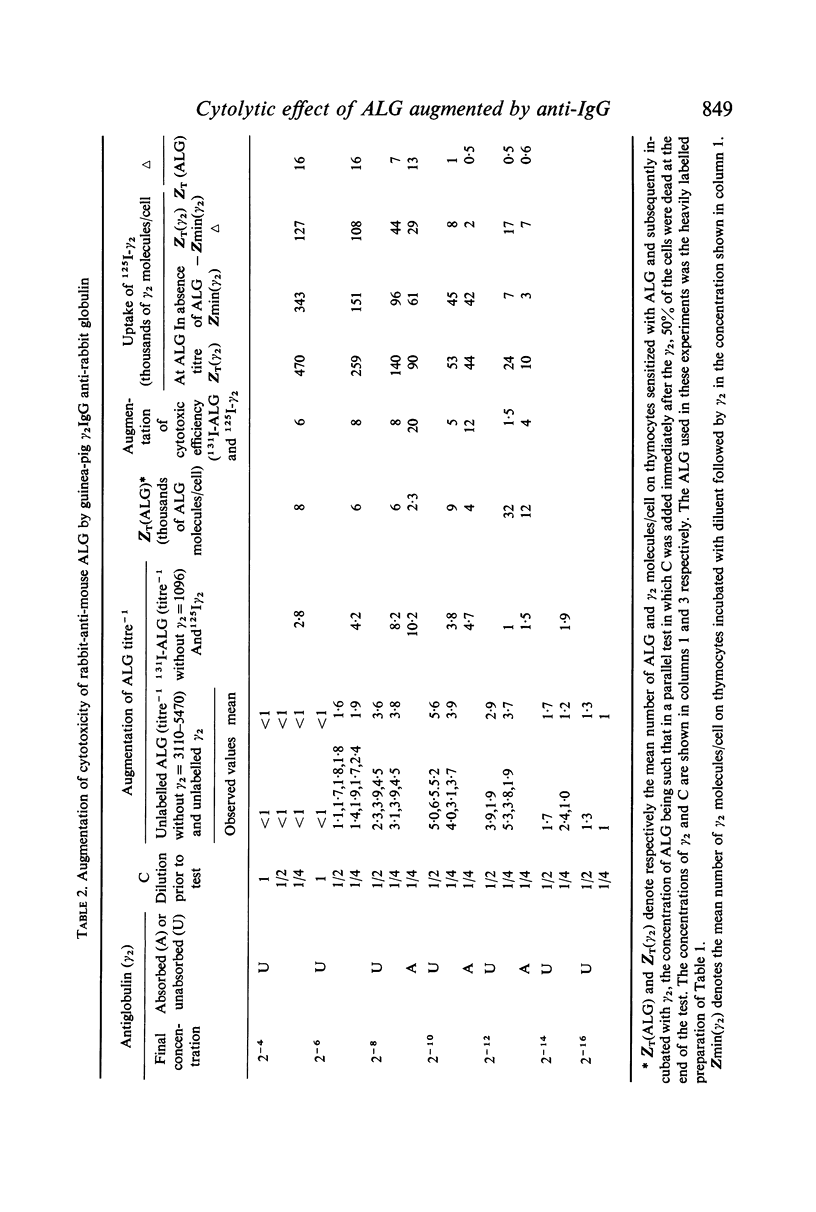


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson N. F., James K., Woodruff M. F. Effect of antilymphocytic antibody and antibody fragments on skin-homograft survival and the blood-lymphocyte count in rats. Lancet. 1967 May 27;1(7500):1126–1128. doi: 10.1016/s0140-6736(67)91706-0. [DOI] [PubMed] [Google Scholar]
- BENACERRAF B., OVARY Z., BLOCH K. J., FRANKLIN E. C. Properties of guinea pig 7S antibodies. I. Electrophoretic separation of two types of guinea pig 7S antibodies. J Exp Med. 1963 Jun 1;117:937–949. doi: 10.1084/jem.117.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Hemolysin titration based on fixation of the activated first component of complement: evidence that one molecule of hemolysin suffices to sensitize an erythrocyte. J Immunol. 1965 Sep;95(3):559–566. [PubMed] [Google Scholar]
- Cohen A., Schlesinger M. Absorption of guinea pig serum with agar. A method for elimination of itscytotoxicity for murine thymus cells. Transplantation. 1970 Jul;10(1):130–132. doi: 10.1097/00007890-197007000-00027. [DOI] [PubMed] [Google Scholar]
- Dresser D. W., Wortis D. H. Use of an antiglobulin serum to detect cells producing antibody with low haemolytic efficiency. Nature. 1965 Nov 27;208(5013):859–861. doi: 10.1038/208859a0. [DOI] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Humphrey J. H. Haemolytic efficiency of rabbit IgG anti-Forssman antibody and its augmentation by anti-rabbit IgG. Nature. 1967 Dec 30;216(5122):1295–1296. doi: 10.1038/2161295a0. [DOI] [PubMed] [Google Scholar]
- Oliveira B., Osler A. G., Siraganian R. P., Sandberg A. L. The biologic activities of guinea pig antibodies. I. Separation of gamma1 and gamma2 immunoglobulins and their participation in allergic reactions of the immediate type. J Immunol. 1970 Feb;104(2):320–328. [PubMed] [Google Scholar]
- Sterzl J., Ríha I. Detection of cells producing 7S antibodies by the plaque technique. Nature. 1965 Nov 27;208(5013):858–859. doi: 10.1038/208858a0. [DOI] [PubMed] [Google Scholar]
- Woodruff M. F. Antilymphocytic serum. Antibiot Chemother. 1969;15:234–249. doi: 10.1159/000386784. [DOI] [PubMed] [Google Scholar]
- Woodruff M., Smith L. H. Cytotoxic efficiency and effect on tumour growth of heterospecific antilymphocytic and antitumour sera. Nature. 1970 Jan 24;225(5230):377–379. doi: 10.1038/225377a0. [DOI] [PubMed] [Google Scholar]


