Abstract
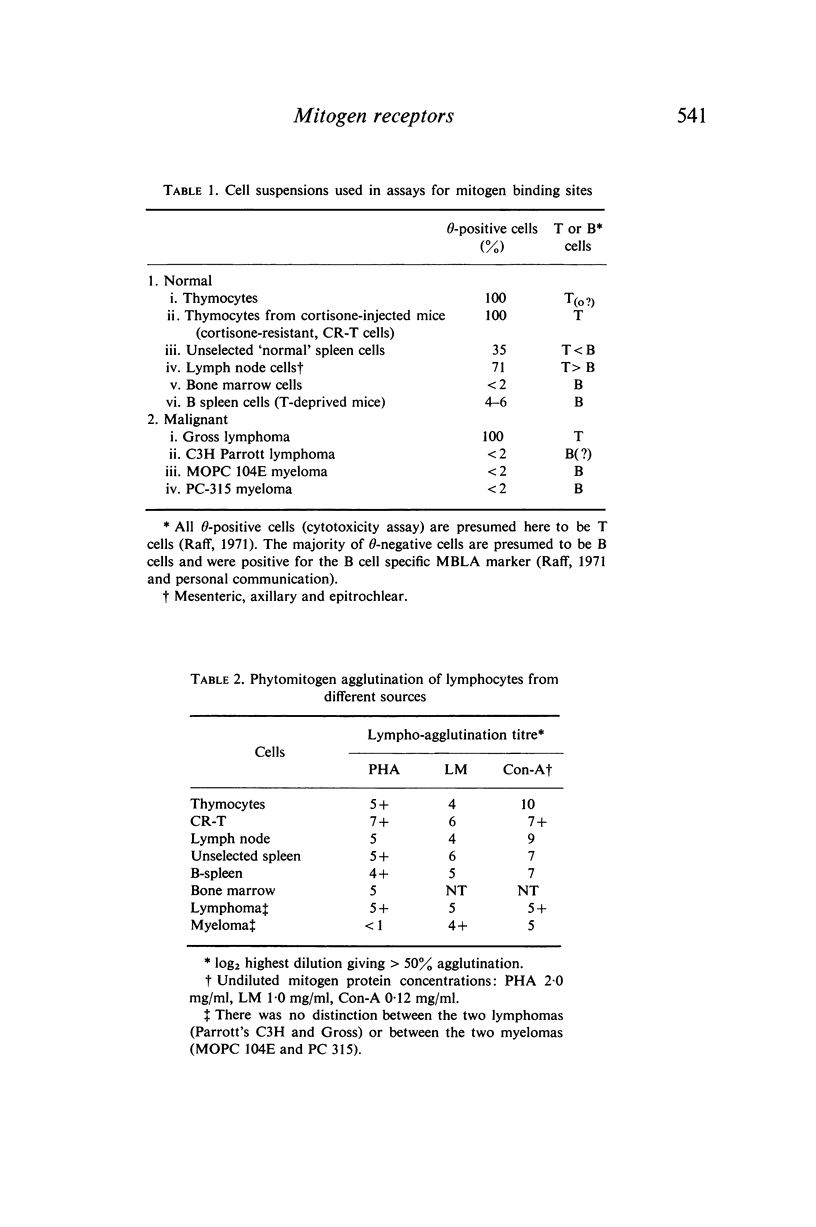
The presence of binding sites for concanavalin A, phytohaemagglutinin and lentil mitogen on the surface of mouse B and T lymphocytes has been investigated in relation to the previously described mitogenic selectivity of these stimulants for T cells. Purified T or B or mixed T/B suspensions of cells have been analysed both for proportions of cells with affinity for phytomitogens and for the relative density of binding sites. In addition, the specificity of mitogens for simple saccharides has been investigated in relation to binding of the former to T and B cells.
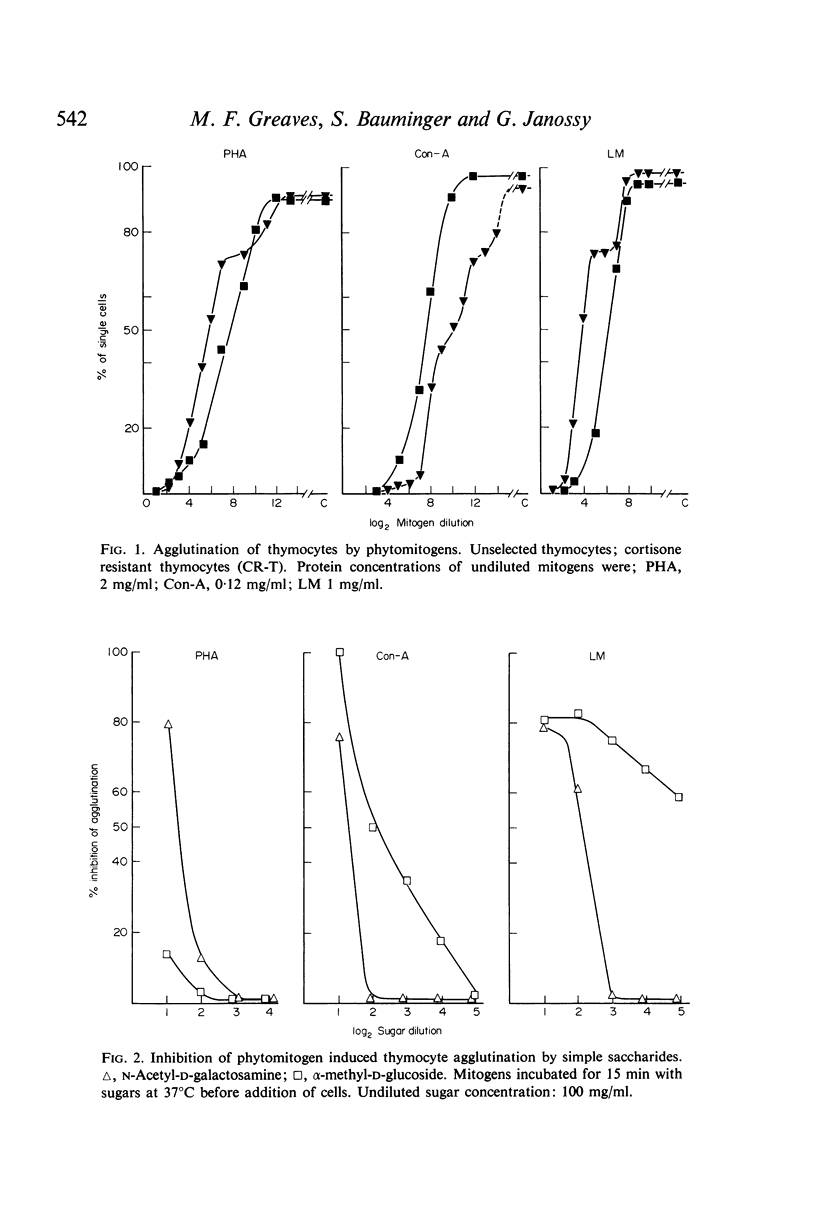
Microscopic agglutination and immunofluorescent studies show that virtually 100% of T and B lymphocytes have binding sites for the three phytomitogens. Quantitative absorption experiments have demonstrated that there is virtually no difference in the relative average density of binding sites on T and B cells.
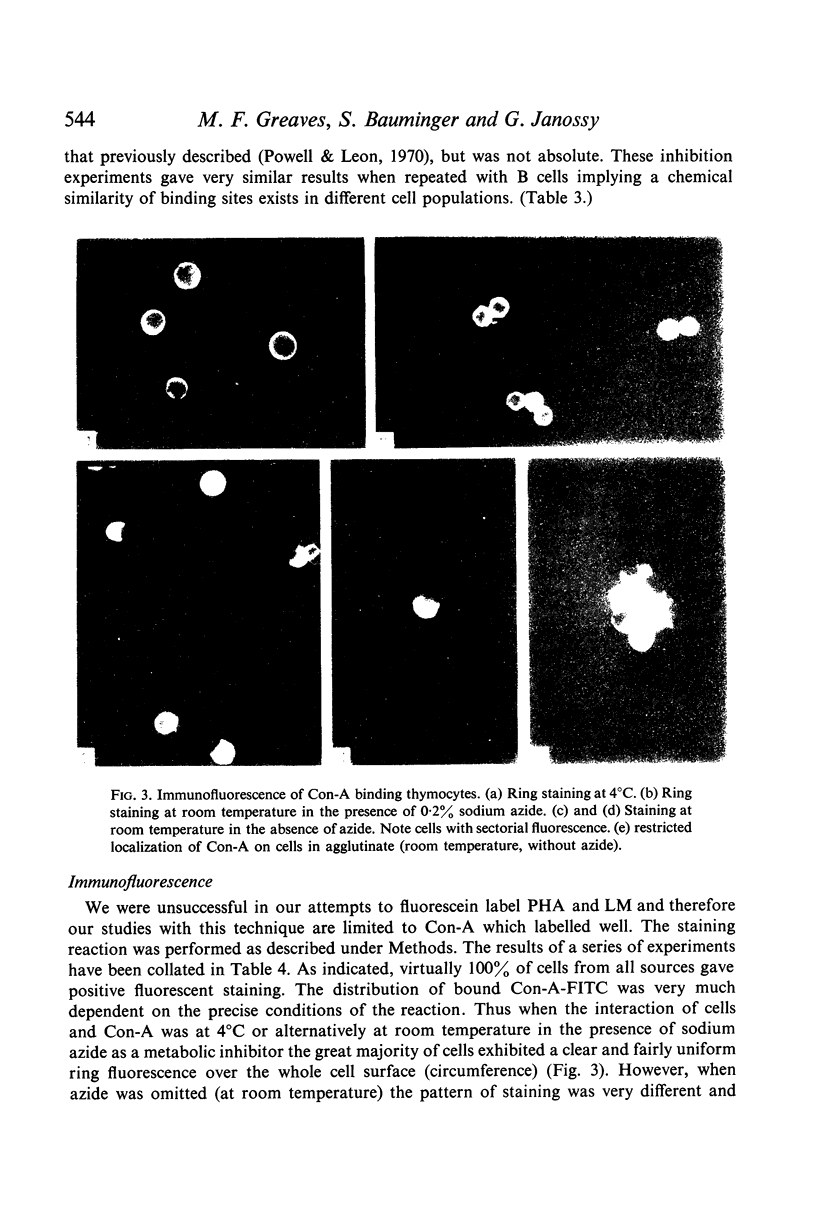
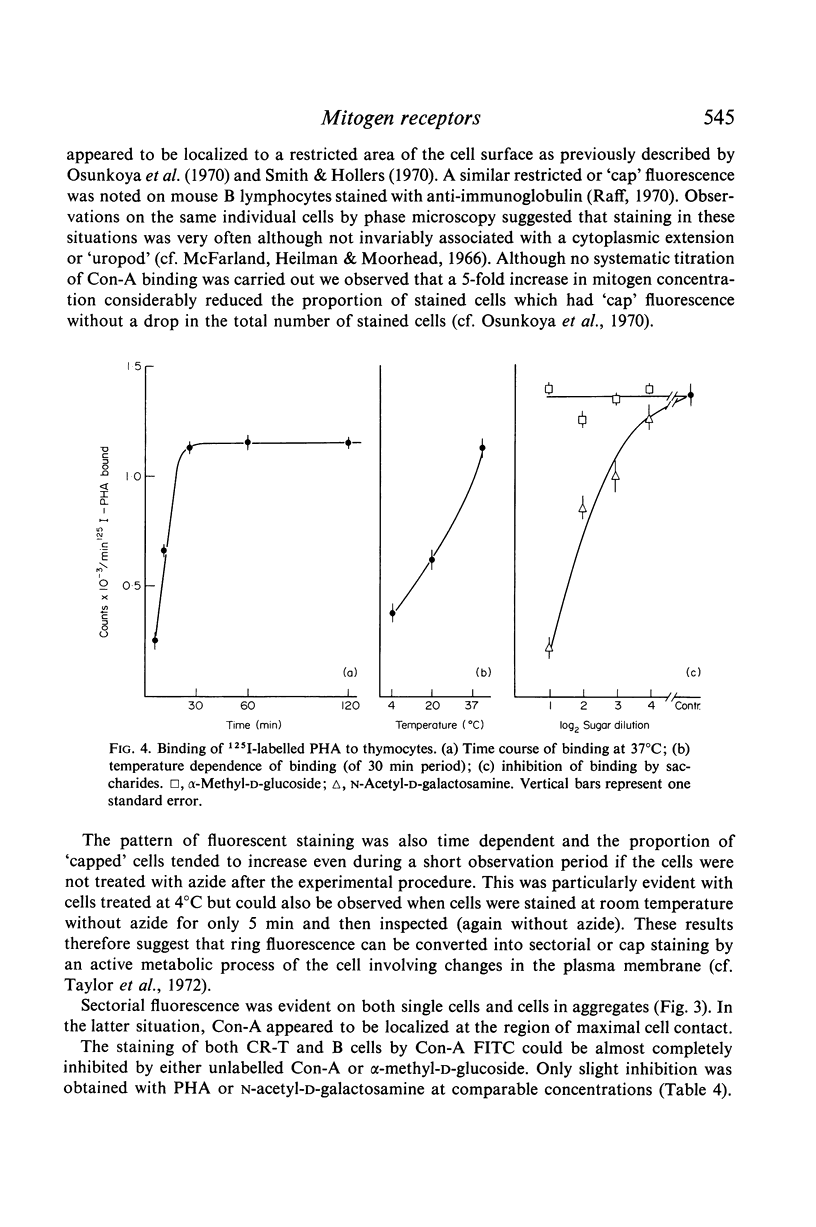
Immunofluorescent studies with Con-A suggested that binding sites are uniformly present over the entire cell surface. However, when lymphocytes are metabolically active an interesting altered localization of bound Con-A molecules to one pole of the cell takes place.
It is suggested that binding sites for the various phytomitogens are qualitatively and topographically distinct on the cell surface but that with respect to any one mitogen T and B cells have qualitatively and quantitatively similar binding sites.
The relevance of these observations to lymphocyte activation is considered.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan D., Auger J., Crumpton M. J. Interaction of phytohemagglutinin with plasma membranes of pig lymphocytes and thymus cells. Exp Cell Res. 1971 Jun;66(2):362–368. doi: 10.1016/0014-4827(71)90689-6. [DOI] [PubMed] [Google Scholar]
- Blomgren H., Andersson B. Characteristics of the immunocompetent cells in the mouse thymus: cell population changes during cortisone-induced atrophy and subsequent regeneration. Cell Immunol. 1970 Nov;1(5):545–560. doi: 10.1016/0008-8749(70)90041-9. [DOI] [PubMed] [Google Scholar]
- Borberg H., Yesner I., Gesner B., Silber R. The effect of N acetyl C-galactosamine and other sugars on the mitogenic activity and attachment of PHA to tonsil cells. Blood. 1968 Jun;31(6):747–757. [PubMed] [Google Scholar]
- Burger M. M., Noonan K. D. Restoration of normal growth by covering of agglutinin sites on tumour cell surface. Nature. 1970 Nov 7;228(5271):512–515. doi: 10.1038/228512a0. [DOI] [PubMed] [Google Scholar]
- Börjeson J., Reisfeld R., Chessin L. N., Welsh P. D., Douglas S. D. Studies on human peripheral blood lymphocytes in vitro. I. Biological and physicochemical properties of the pokeweed mitogen. J Exp Med. 1966 Nov 1;124(5):859–872. doi: 10.1084/jem.124.5.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline M. J., Livingston D. C. Binding of 3 H-concanavalin A by normal and transformed cells. Nat New Biol. 1971 Aug 4;232(31):155–156. doi: 10.1038/newbio232155a0. [DOI] [PubMed] [Google Scholar]
- Curtis A. S., Greaves M. F. The inhibition of cell aggregation by a pure serum protein. J Embryol Exp Morphol. 1965 Jun;13(3):309–326. [PubMed] [Google Scholar]
- Dent P. B. Inhibition by phytohemagglutinin of DNA synthesis in cultured mouse lymphomas. J Natl Cancer Inst. 1971 Apr;46(4):763–773. [PubMed] [Google Scholar]
- Fanger M. W., Hart D. A., Wells J. V., Nisonoff A. Requirement for cross-linkage in the stimulation of transformation of rabbit peripheral lymphocytes by antiglobulin reagents. J Immunol. 1970 Dec;105(6):1484–1492. [PubMed] [Google Scholar]
- Harris R., Ukaejiofo E. O. Rapid preparation of lymphocytes for tissue-typing. Lancet. 1969 Aug 9;2(7615):327–327. doi: 10.1016/s0140-6736(69)90096-8. [DOI] [PubMed] [Google Scholar]
- Holland N. H., Holland P. Haemagglutinating, precipitating and lymphocyte-stimulating factors of phytohaemagglutinin. Nature. 1965 Sep 18;207(5003):1307–1308. doi: 10.1038/2071307a0. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Jones G., Torrigiani G., Roitt I. M. Immunoglobulin determinants on mouse lymphocytes. J Immunol. 1971 Jun;106(6):1425–1430. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. The structure of a phytohemagglutinin receptor site from human erythrocytes. J Biol Chem. 1970 May 25;245(10):2536–2545. [PubMed] [Google Scholar]
- Kornfeld S., Kornfeld R. Solubilization and partial characterization of a phytohemagglutinin receptor site from human erythrocytes. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1439–1446. doi: 10.1073/pnas.63.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy M., Chessin L. N. The effect of plant mitogens on humoral and cellular immune responses. Antibiot Chemother. 1969;15:199–212. doi: 10.1159/000386782. [DOI] [PubMed] [Google Scholar]
- Leon M. A. Concanavalin A reaction with human normal immunoglobulin G and myeloma immunoglobulin G. Science. 1967 Dec 8;158(3806):1325–1326. doi: 10.1126/science.158.3806.1325. [DOI] [PubMed] [Google Scholar]
- Lindahl-Kiessling K., Mattsson A. Mechanism of phytohemagglutinin (PHA) action. IV. Effect of some metabolic inhibitors on binding of PHA to lymphocytes and the stimulatory potential of PHA-pretreated cells. Exp Cell Res. 1971 Apr;65(2):307–312. doi: 10.1016/0014-4827(71)90006-1. [DOI] [PubMed] [Google Scholar]
- McFarland W., Heilman D. H., Moorhead J. F. Functional anatomy of the lymphocyte in immunological reactions in vitro. J Exp Med. 1966 Nov 1;124(5):851–858. doi: 10.1084/jem.124.5.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse J. H. Immunological studies of phytohaemagglutinin. I. Reaction between phytohaemagglutinin and normal sera. Immunology. 1968 May;14(5):713–724. [PMC free article] [PubMed] [Google Scholar]
- Ozanne B., Sambrook J. Binding of radioactively labelled concanavalin A and wheat germ agglutinin to normal and virus-transformed cells. Nat New Biol. 1971 Aug 4;232(31):156–160. doi: 10.1038/newbio232156a0. [DOI] [PubMed] [Google Scholar]
- Powell A. E., Leon M. A. Reversible interaction of human lymphocytes with the mitogen concanavalin A. Exp Cell Res. 1970 Oct;62(2):315–325. doi: 10.1016/0014-4827(70)90560-4. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Raff M. C. Two distinct populations of peripheral lymphocytes in mice distinguishable by immunofluorescence. Immunology. 1970 Oct;19(4):637–650. [PMC free article] [PubMed] [Google Scholar]
- Raff M. Theta isoantigen as a marker of thymus-derived lymphocytes in mice. Nature. 1969 Oct 25;224(5217):378–379. doi: 10.1038/224378a0. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Hollers J. C. The pattern of binding of fluorescein-labeled concanavalin A to the motile lymphocyte. J Reticuloendothel Soc. 1970 Nov;8(5):458–464. [PubMed] [Google Scholar]
- Young N. M., Leon M. A., Takahashi T., Howard I. K., Sage H. J. Studies on a phytohemagglutinin from the lentil. 3. Reaction of Lens culinaris hemagglutinin with polysaccharides, glycoproteins, and lymphocytes. J Biol Chem. 1971 Mar 25;246(6):1596–1601. [PubMed] [Google Scholar]


