Abstract
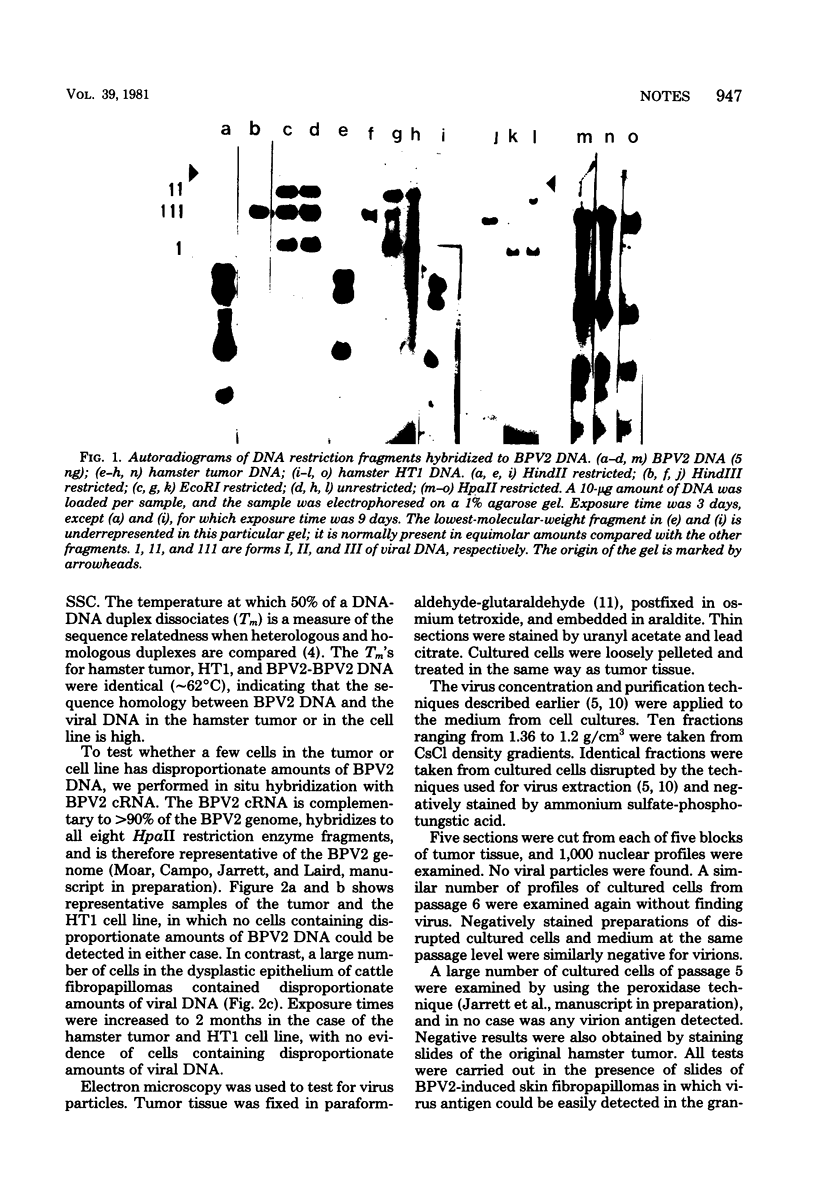
A fibrosarcoma was induced in a hamster by bovine papilloma virus type 2 (BPV2). The content of BPV2 DNA sequences was measured by DNA-DNA and cRNA-DNA hybridizations. The tumor contained approximately 300 BPV2 genome equivalents per cell. Southern blot hybridization indicated that the viral DNA was in free form, the entire genome most likely being present. In situ hybridization with BPV2 cRNA showed that multiple genome copies were present in each cell. Neither virus particles nor virus coat antigens could be detected in the tumor. A cell line was established from the fibrosarcoma, and the cells contained multiple copies of the BPV2 genome. The latter was in free form, and all of the DNA sequences appeared to be present in multiple copies and in all cells. An extensive search failed to reveal the presence of virus or viral antigens.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amtmann E., Müller H., Sauer G. Equine connective tissue tumors contain unintegrated bovine papilloma virus DNA. J Virol. 1980 Sep;35(3):962–964. doi: 10.1128/jvi.35.3.962-964.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLACK P. H., HARTLEY J. W., ROWE W. P., HUEBNER R. J. TRANSFORMATION OF BOVINE TISSUE CULTURE CELLS BY BOVINE PAPILLOMA VIRUS. Nature. 1963 Sep 7;199:1016–1018. doi: 10.1038/1991016a0. [DOI] [PubMed] [Google Scholar]
- BOIRON M., LEVY J. P., THOMAS M., FRIEDMANN J. C., BERNARD J. SOME PROPERTIES OF BOVINE PAPILLOMA VIRUS. Nature. 1964 Jan 25;201:423–424. doi: 10.1038/201423a0. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Cheville N. F. Studies on connective tissue tumors in the hamster produced by bovine papilloma virus. Cancer Res. 1966 Nov;26(11):2334–2339. [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gordon D. E., Olson C. Meningiomas and fibroblastic neoplasia in calves induced with the bovine papilloma virus. Cancer Res. 1968 Dec;28(12):2423–2431. [PubMed] [Google Scholar]
- Jarrett W. F., Murphy J., O'Neil B. W., Laird H. M. Virus-induced papillomas of the alimentary tract of cattle. Int J Cancer. 1978 Sep 15;22(3):323–328. doi: 10.1002/ijc.2910220316. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D. Apparent lack of integration of bovine papillomavirus DNA in virus-induced equine and bovine tumor cells and virus-transformed mouse cells. Virology. 1981 Jan 30;108(2):251–255. doi: 10.1016/0042-6822(81)90433-5. [DOI] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C., Meinke W. Bovine papilloma virus: presence of virus-specific DNA sequences in naturally occurring equine tumors. Proc Natl Acad Sci U S A. 1977 Feb;74(2):524–528. doi: 10.1073/pnas.74.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D., Olson C., Meinke W. Quantitation of bovine papilloma viral DNA in viral-induced tumors. J Virol. 1976 Mar;17(3):824–831. doi: 10.1128/jvi.17.3.824-831.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D. Physical maps of bovine papillomavirus type 1 and type 2 genomes. J Virol. 1979 Nov;32(2):684–687. doi: 10.1128/jvi.32.2.684-687.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster W. D., Theilen G. H., Olson C. Hybridization of bovine papilloma virus type 1 and type 2 DNA to DNA from virus-induced hamster tumors and naturally occurring equine tumors. Intervirology. 1979;11(4):227–233. doi: 10.1159/000149038. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The determination of the molecular weight of ribonucleic acid by polyacrylamide-gel electrophresis. The effects of changes in conformation. Biochem J. 1969 Jun;113(1):131–138. doi: 10.1042/bj1130131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moar M. H., Klein G. Detection of Epstein-Barr virus (EBV) DNA sequences using in situ hybridization. Biochim Biophys Acta. 1978 Jun 22;519(1):49–64. doi: 10.1016/0005-2787(78)90061-8. [DOI] [PubMed] [Google Scholar]
- OLSON C., Jr, COOK R. H. Cutaneous sarcoma-like lesions of the horse caused by the agent of bovine papilloma. Proc Soc Exp Biol Med. 1951 Jun;77(2):281–284. doi: 10.3181/00379727-77-18750. [DOI] [PubMed] [Google Scholar]
- Olson C., Gordon D. E., Robl M. G., Lee K. P. Oncogenicity of bovine papilloma virus. Arch Environ Health. 1969 Dec;19(6):827–837. doi: 10.1080/00039896.1969.10666938. [DOI] [PubMed] [Google Scholar]
- Orth G., Jeanteur P., Croissant O. Evidence for and localization of vegetative viral DNA replication by autoradiographic detection of RNA-DNA hybrids in sections of tumors induced by Shope papilloma virus. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1876–1880. doi: 10.1073/pnas.68.8.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saveria Campo M., Moar M. H., Jarrett W. F., Laird H. M. A new papillomavirus associated with alimentary cancer in cattle. Nature. 1980 Jul 10;286(5769):180–182. doi: 10.1038/286180a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]