Abstract
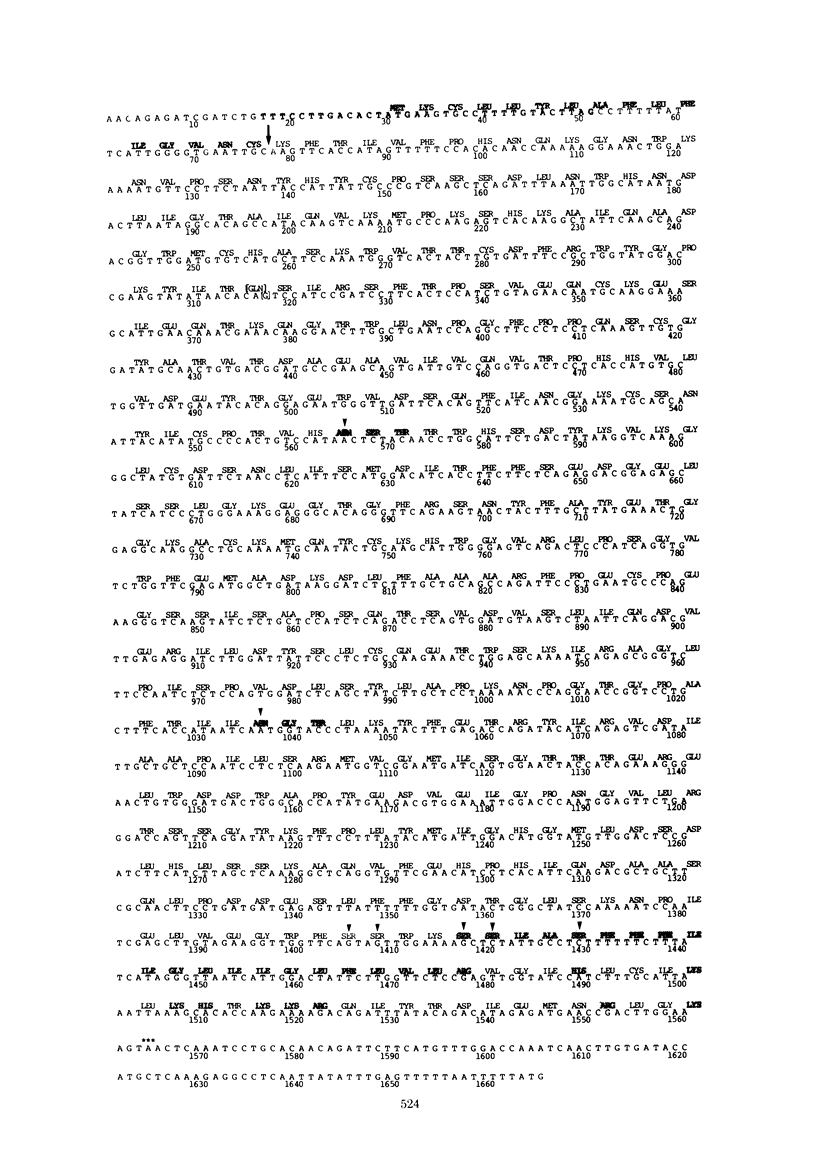
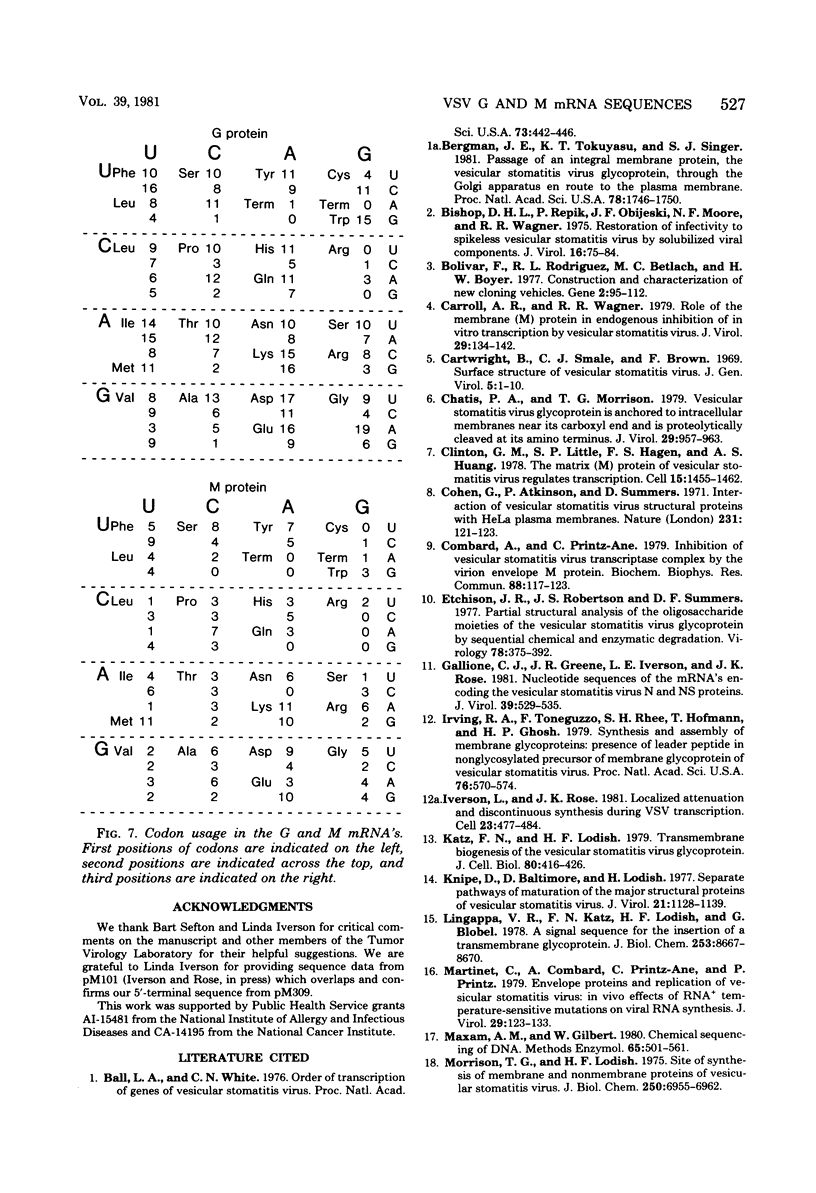
The complete nucleotide sequences of the vesicular stomatitis virus mRNA's encoding the glycoprotein (G) and the matrix protein (M) have been determined from cDNA clones that contain the complete coding sequences from each mRNA. The G protein mRNA is 1,665 nucleotides long, excluding polyadenylic acid, and encodes a protein of 511 amino acids including a signal peptide of 16 amino acids. G protein contains two large hydrophobic domains, one in the signal peptide and the other in the transmembrane segment near the COOH terminus. Two sites of glycosylation are predicted at amino acid residues 178 and 335. The close correspondence of the positions of these sites with the reported timing of the addition of the two oligosaccharides during synthesis of G suggests that glycosylation occurs as soon as the appropriate asparagine residues traverse the membrane of the rough endoplasmic reticulum. The mRNA encoding the vesicular stomatitis virus M protein is 831 nucleotides long, excluding polyadenylic acid, and encodes a protein of 229 amino acids. The predicted M protein sequence does not contain any long hydrophobic or nonpolar domains that might promote membrane association. The protein is rich in basic amino acids and contains a highly basic amino terminal domain. Details of construction of the nearly full-length cDNA clones are presented.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. H., Repik P., Obijeski J. F., Moore N. F., Wagner R. R. Restitution of infectivity to spikeless vesicular stomatitis virus by solubilized viral components. J Virol. 1975 Jul;16(1):75–84. doi: 10.1128/jvi.16.1.75-84.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Role of the membrane (M) protein in endogenous inhibition of in vitro transcription by vesicular stomatitis virus. J Virol. 1979 Jan;29(1):134–142. doi: 10.1128/jvi.29.1.134-142.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright B., Smale C. J., Brown F. Surface structure of vesicular stomatitis virus. J Gen Virol. 1969 Jul;5(1):1–10. doi: 10.1099/0022-1317-5-1-1. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Vesicular stomatitis virus glycoprotein is anchored to intracellular membranes near its carboxyl end and is proteolytically cleaved at its amino terminus. J Virol. 1979 Mar;29(3):957–963. doi: 10.1128/jvi.29.3.957-963.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Little S. P., Hagen F. S., Huang A. S. The matrix (M) protein of vesicular stomatitis virus regulates transcription. Cell. 1978 Dec;15(4):1455–1462. doi: 10.1016/0092-8674(78)90069-7. [DOI] [PubMed] [Google Scholar]
- Cohen G. H., Atkinson P. H., Summers D. F. Interactions of vesicular stomatitis virus structural proteins with HeLa plasma membranes. Nat New Biol. 1971 May 26;231(21):121–123. doi: 10.1038/newbio231121a0. [DOI] [PubMed] [Google Scholar]
- Combard A., Printz Ane C. Inhibition of vesicular stomatitis virus transcriptase complex by the virion envelope M protein. Biochem Biophys Res Commun. 1979 May 14;88(1):117–123. doi: 10.1016/0006-291x(79)91704-2. [DOI] [PubMed] [Google Scholar]
- Etchison J. R., Robertson J. S., Summers D. F. Partial structural analysis of the oligosaccharide moieties of the vesicular stomatitis virus glycoprotein by sequential chemical and enzymatic degradation. Virology. 1977 May 15;78(2):375–392. doi: 10.1016/0042-6822(77)90115-5. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving R. A., Toneguzzo F., Rhee S. H., Hofmann T., Ghosh H. P. Synthesis and assembly of membrane glycoproteins: presence of leader peptide in nonglycosylated precursor of membrane glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):570–574. doi: 10.1073/pnas.76.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson L. E., Rose J. K. Localized attenuation and discontinuous synthesis during vesicular stomatitis virus transcription. Cell. 1981 Feb;23(2):477–484. doi: 10.1016/0092-8674(81)90143-4. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Lodish H. F. Transmembrane biogenesis of the vesicular stomatitis virus glycoprotein. J Cell Biol. 1979 Feb;80(2):416–426. doi: 10.1083/jcb.80.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingappa V. R., Katz F. N., Lodish H. F., Blobel G. A signal sequence for the insertion of a transmembrane glycoprotein. Similarities to the signals of secretory proteins in primary structure and function. J Biol Chem. 1978 Dec 25;253(24):8667–8670. [PubMed] [Google Scholar]
- Martinet C., Combard A., Printz-Ané C., Printz P. Envelope proteins and replication of vesicular stomatitis virus: in vivo effects of RNA+ temperature-sensitive mutations on viral RNA synthesis. J Virol. 1979 Jan;29(1):123–133. doi: 10.1128/jvi.29.1.123-133.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Ohmori H., Tomizawa J. I., Maxam A. M. Detection of 5-methylcytosine in DNA sequences. Nucleic Acids Res. 1978 May;5(5):1479–1485. doi: 10.1093/nar/5.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C. L., Penhoet E. E., Ballou C. E. Carbohydrate structure of vesicular stomatitis virus glycoprotein. J Biol Chem. 1978 Aug 25;253(16):5600–5612. [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete sequences of the ribosome recognition sites in vesicular stomatitis virus mRNAs: recognition by the 40S and 80S complexes. Cell. 1978 Jun;14(2):345–353. doi: 10.1016/0092-8674(78)90120-4. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Iverson L. Nucleotide sequences from the 3'-ends of vesicular stomatitis virus mRNA's as determined from cloned DNA. J Virol. 1979 Nov;32(2):404–411. doi: 10.1128/jvi.32.2.404-411.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Lodish H. F., Brock M. L. Giant heterogeneous polyadenylic acid on vesicular stomatitis virus mRNA synthesized in vitro in the presence of S-adenosylhomocysteine. J Virol. 1977 Feb;21(2):683–693. doi: 10.1128/jvi.21.2.683-693.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Nucleotide sequences of ribosome recgonition sites in messenger RNAs of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3672–3676. doi: 10.1073/pnas.74.9.3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Welch W. J., Sefton B. M., Esch F. S., Ling N. C. Vesicular stomatitis virus glycoprotein is anchored in the viral membrane by a hydrophobic domain near the COOH terminus. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3884–3888. doi: 10.1073/pnas.77.7.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Fine R. E. Coated vesicles transport newly synthesized membrane glycoproteins from endoplasmic reticulum to plasma membrane in two successive stages. Proc Natl Acad Sci U S A. 1980 Feb;77(2):780–784. doi: 10.1073/pnas.77.2.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SWARTZ M. N., TRAUTNER T. A., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. XI. Further studies on nearest neighbor base sequences in deoxyribonucleic acids. J Biol Chem. 1962 Jun;237:1961–1967. [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Staden R. Sequence data handling by computer. Nucleic Acids Res. 1977 Nov;4(11):4037–4051. doi: 10.1093/nar/4.11.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Synthesis and glycosylation in vitro of glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1516–1520. doi: 10.1073/pnas.74.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wensink P. C., Finnegan D. J., Donelson J. E., Hogness D. S. A system for mapping DNA sequences in the chromosomes of Drosophila melanogaster. Cell. 1974 Dec;3(4):315–325. doi: 10.1016/0092-8674(74)90045-2. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. doi: 10.1093/nar/8.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]