Abstract
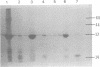
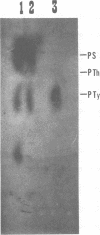
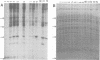
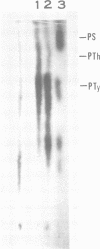
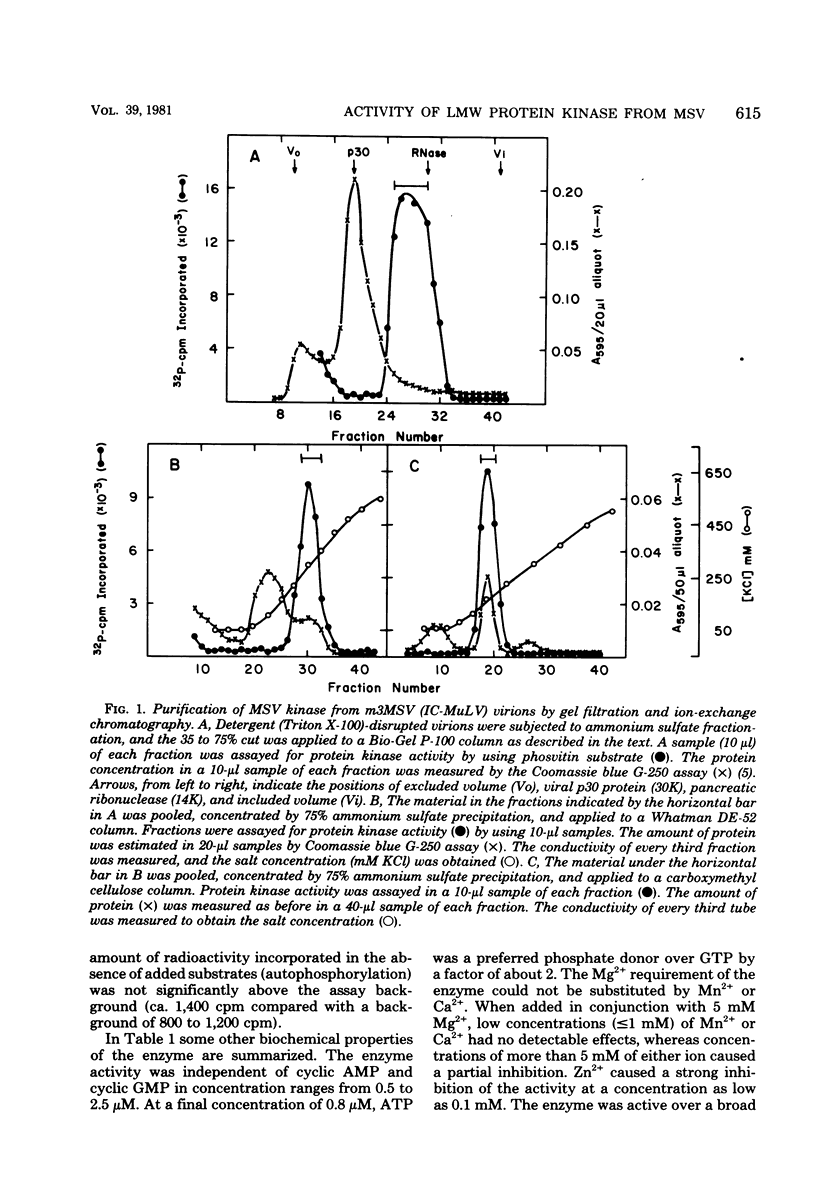
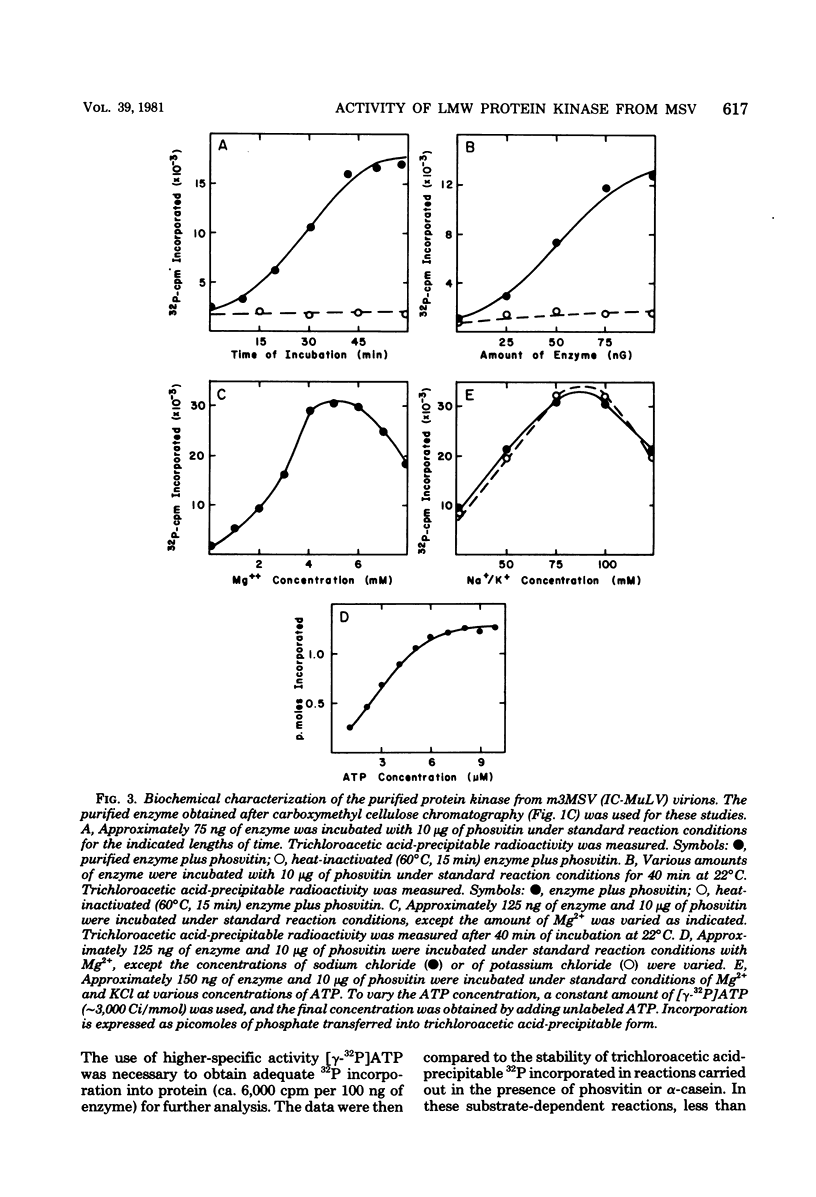
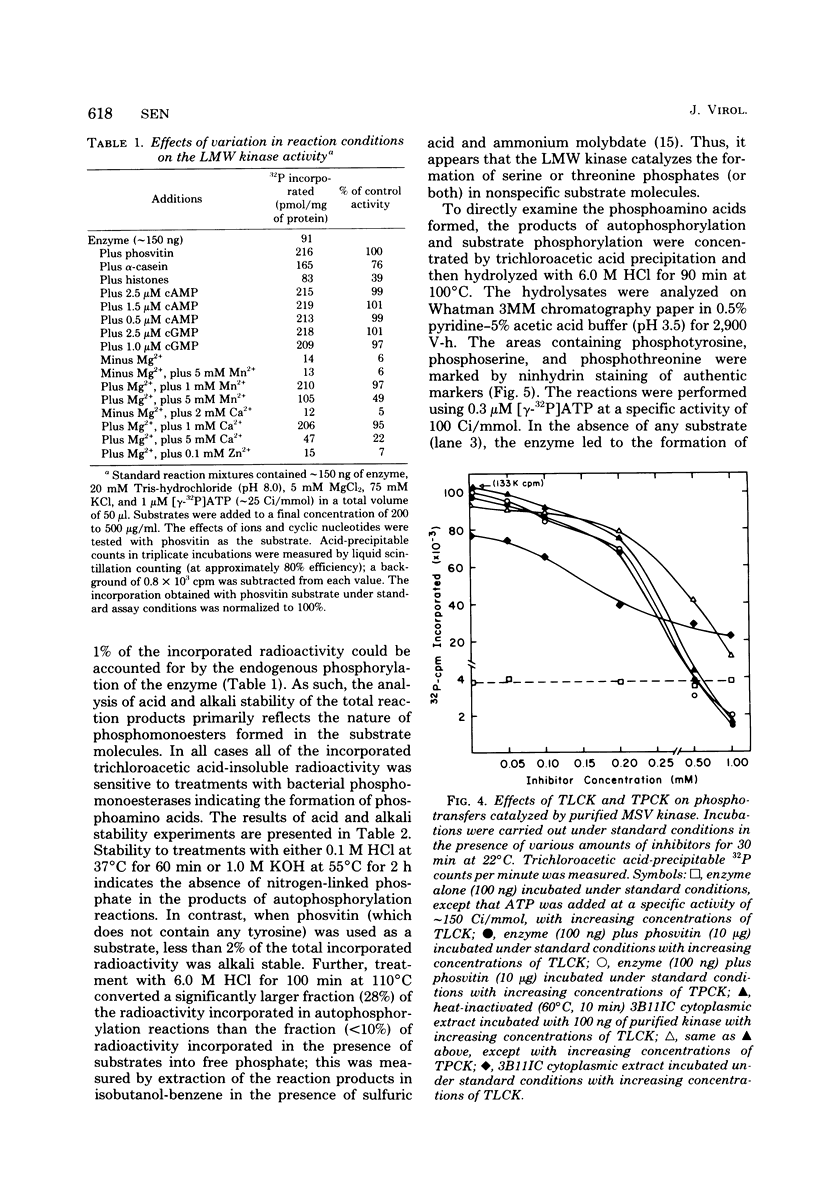
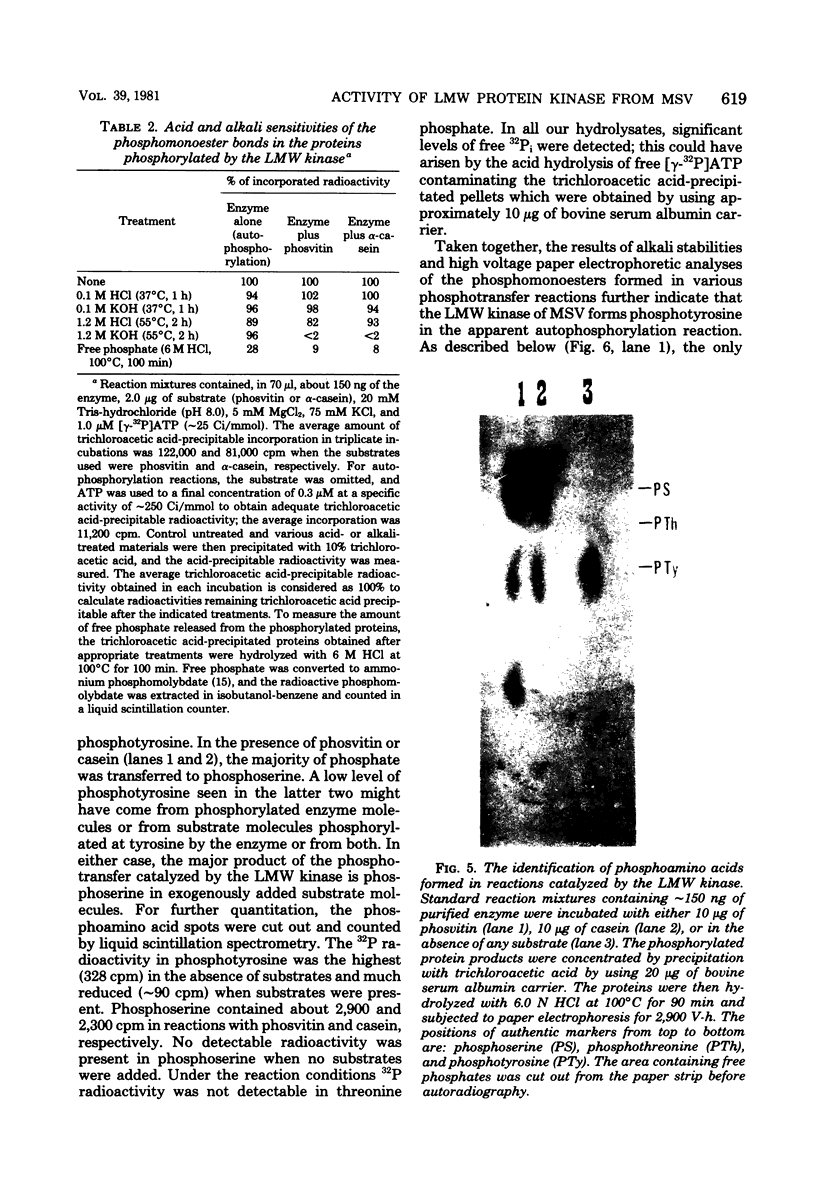
The low-molecular-weight (LMW) protein kinase associated with high-titer murine sarcoma virions have been extensively purified by ammonium sulfate fractionation. Bio-Gel P-100 gel filtration, DEAE-cellulose and carboxymethyl cellulose chromatography. The purified enzyme migrates as a 16K polypeptide in polyacrylamide gel electrophoresis in the presence of sodium dodecyl sulfate. The enzyme catalyzes phosphotransfer with ATP as a phosphate donor to various exogenously added proteins as acceptors; it requires Mg2+ and is independent of cyclic AMP. The enzyme preparation catalyzes a low level of phosphorylation in the absence of any exogenously added substrate and forms phosphotyrosine. However, in the presence of acceptor protein molecules including total soluble cytoplasmic proteins of murine sarcoma virus-transformed mouse cells, the phosphorylated end products contain predominantly phosphoserine. The virion-associated enzyme also shows a preference for phosphorylating certain polypeptides in the soluble cytoplasmic extracts of murine sarcoma virus-transformed cells.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassin R. H., Tuttle N., Fischinger P. J. Isolation of murine sarcoma virus-transformed mouse cells which are negative for leukemia virus from agar suspension cultures. Int J Cancer. 1970 Jul 15;6(1):95–107. doi: 10.1002/ijc.2910060114. [DOI] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitte L., Kabat D. Isotopic labeling and analysis of phosphoproteins from mammalian ribosomes. Methods Enzymol. 1974;30:563–590. doi: 10.1016/0076-6879(74)30056-0. [DOI] [PubMed] [Google Scholar]
- Blair D. G., Hull M. A., Finch E. A. The isolation and preliminary characterization of temperature-sensitive transformation mutants of Moloney sarcoma virus. Virology. 1979 Jun;95(2):303–316. doi: 10.1016/0042-6822(79)90486-0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Sharp P. A., Weinberg R. A. Comparative study of different isolates of murine sarcoma virus. J Virol. 1979 Dec;32(3):1015–1027. doi: 10.1128/jvi.32.3.1015-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischinger P. J., Moore C. O., O'Connor T. E. Isolation and identification of a helper virus found in the Moloney sarcoma-leukemia virus complex. J Natl Cancer Inst. 1969 Apr;42(4):605–622. [PubMed] [Google Scholar]
- Gumport R. I., Lehman I. R. Structure of the DNA ligase-adenylate intermediate: lysine (epsilon-amino)-linked adenosine monophosphoramidate. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2559–2563. doi: 10.1073/pnas.68.10.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hokin L. E., Sastry P. S., Galsworthy P. R., Yoda A. Evidence that a phosphorylated intermediate in a brain transport adenosine triphosphatase is an acyl phosphate. Proc Natl Acad Sci U S A. 1965 Jul;54(1):177–184. doi: 10.1073/pnas.54.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamine J., Burr J. G., Buchanan J. M. Multiple forms of sarc gene proteins from Rous sarcoma virus RNA. Proc Natl Acad Sci U S A. 1978 Jan;75(1):366–370. doi: 10.1073/pnas.75.1.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levinson A. D., Oppermann H., Levintow L., Varmus H. E., Bishop J. M. Evidence that the transforming gene of avian sarcoma virus encodes a protein kinase associated with a phosphoprotein. Cell. 1978 Oct;15(2):561–572. doi: 10.1016/0092-8674(78)90024-7. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Hunter T., Beemon K. In vitro translation of virion RNA from Moloney murine sarcoma virus. Virology. 1980 Feb;101(1):91–103. doi: 10.1016/0042-6822(80)90486-9. [DOI] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Richert N., Davies P. J., Jay G., Pastan I. Inhibition of the transformation-specific kinase in ASV-transformed cells by N-alpha-tosyl-L-lysyl chloromethyl ketone. Cell. 1979 Oct;18(2):369–374. doi: 10.1016/0092-8674(79)90056-4. [DOI] [PubMed] [Google Scholar]
- Rübsamen H., Friis R. R., Bauer H. Src Gene product from different strains of avian sarcoma virus: Kinetics and possible mechanism of heat inactivation of protein kinase activity from cells infected by transformation-defective, temperature-sensitive mutant and wild-type virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):967–971. doi: 10.1073/pnas.76.2.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A., Todaro G. J. A murine sarcoma virus-associated protein kinase: interaction with actin and microtubular protein. Cell. 1979 Jun;17(2):347–356. doi: 10.1016/0092-8674(79)90161-2. [DOI] [PubMed] [Google Scholar]
- Sen A., Todaro G. J., Blair D. G., Robey W. G. Thermolabile protein kinase molecules in a temperature-sensitive murine sarcoma virus pseudotype. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3617–3621. doi: 10.1073/pnas.76.8.3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J. Inhibition of protease activity in cultures of rous sarcoma virus-transformed cells: effect on the transformed phenotype. Cell. 1975 Jul;5(3):253–261. doi: 10.1016/0092-8674(75)90100-2. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Sun L., Rosenberg N., Baltimore D. A trans-acting protein kinase identified in cells transformed by Abelson murine leukemia virus. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):855–857. doi: 10.1101/sqb.1980.044.01.091. [DOI] [PubMed] [Google Scholar]