Abstract
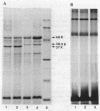
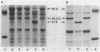
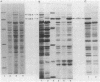
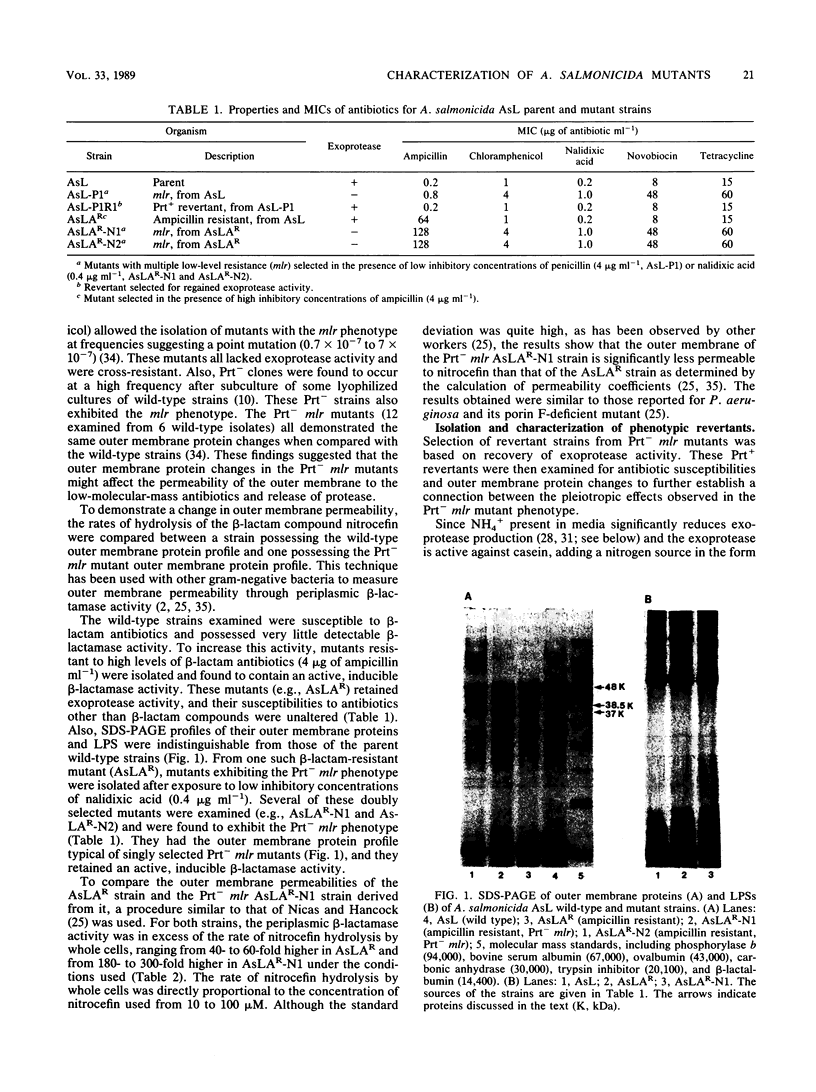
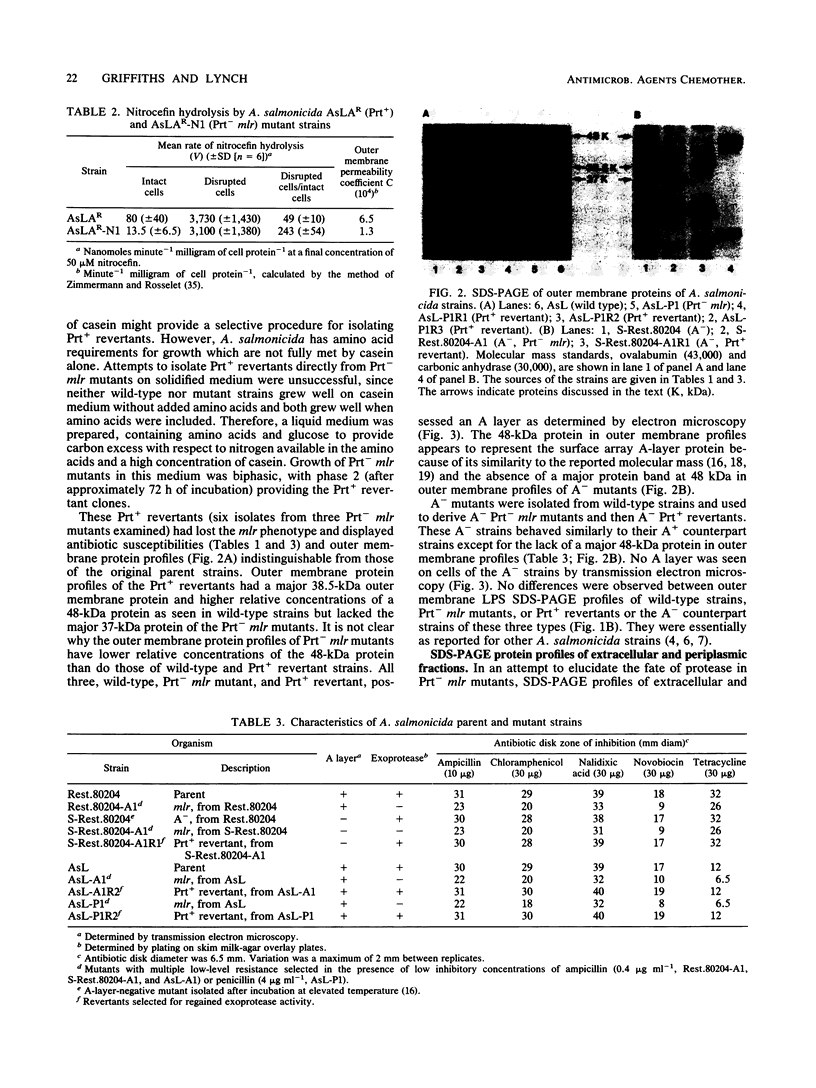
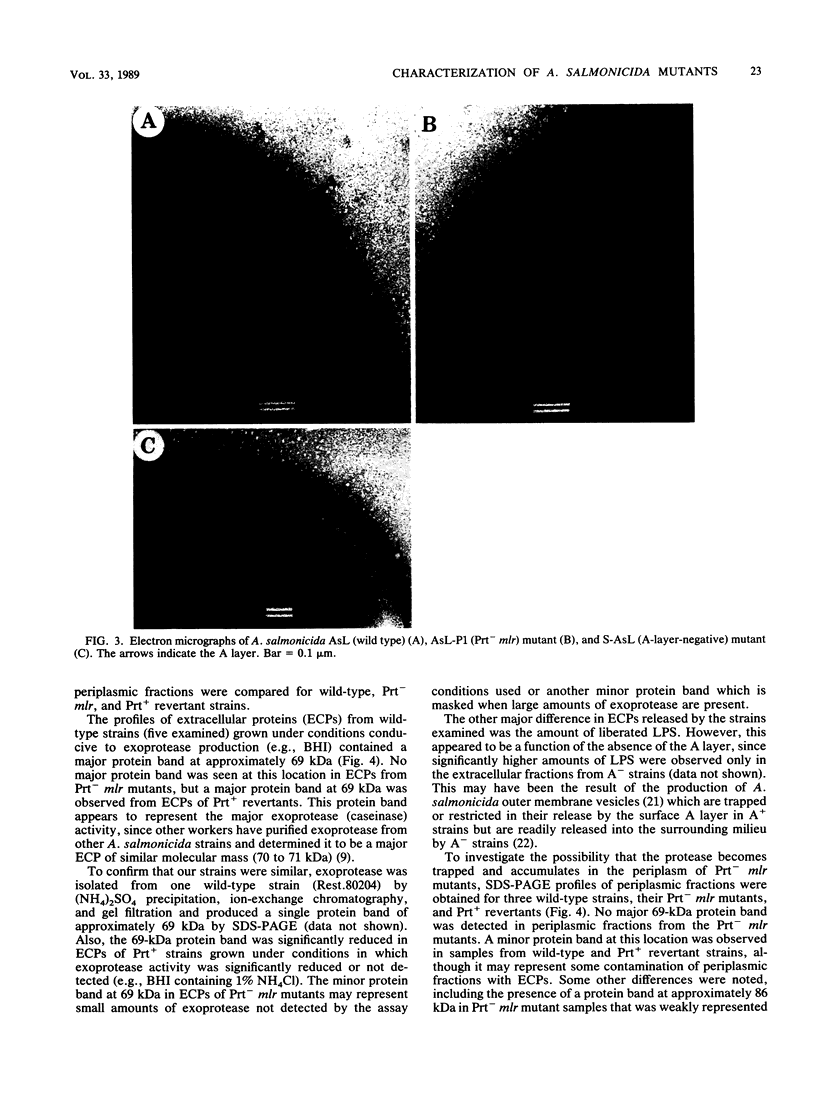
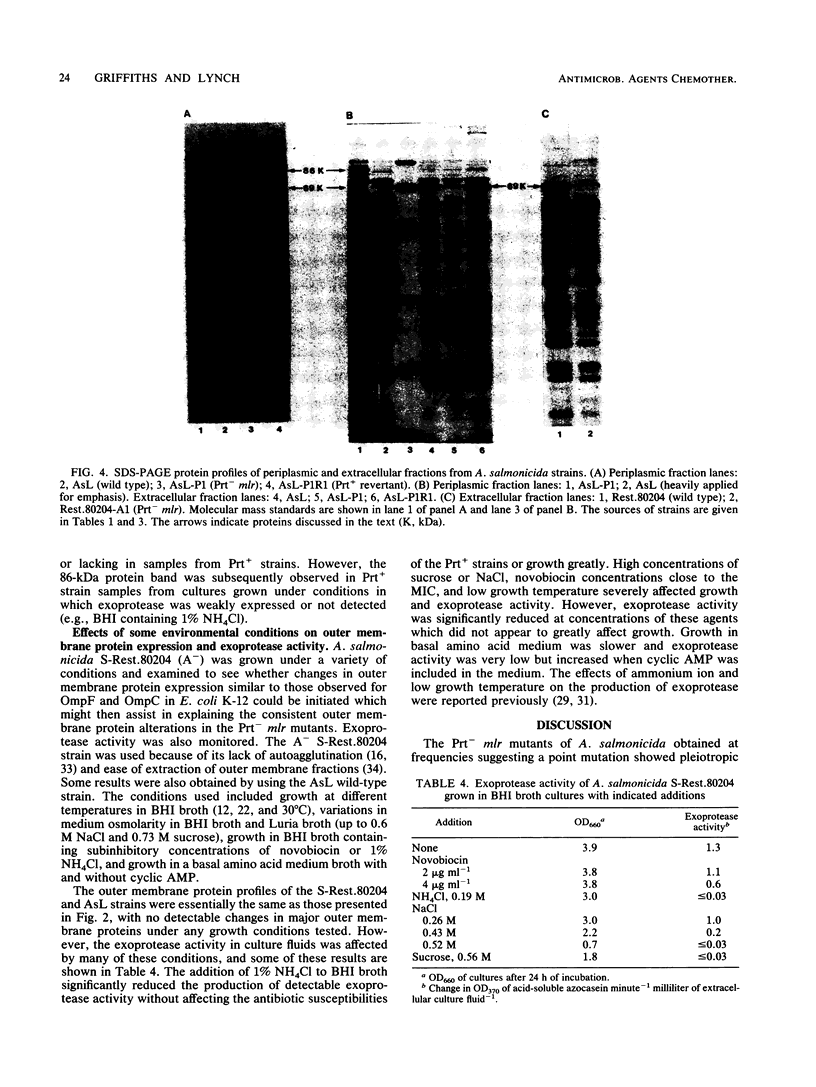
Aeromonas salmonicida mutants selected for low-level resistance to small-molecular-mass antibiotics occur at frequencies that suggest point mutations and exhibit pleiotropic effects such as a multiple low-level antibiotic resistance, changes in outer membrane protein profiles, and loss of major exoprotease activity. Multiple low-level resistance appeared as the result of decreased outer membrane permeability associated with a change from a 38.5- to a 37-kilodalton (kDa) outer membrane protein. This decreased outer membrane permeability was determined by rates of nitrocefin hydrolysis by periplasmic beta-lactamase activity. The findings described above were supported by isolation of revertant strains selected for regained exoprotease activity, which also lost multiple low-level resistance and possessed outer membrane protein profiles indistinguishable from those of the original parent strains. Exoprotease from parent and revertant strains was identified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis as a major extracellular protein of approximately 69 kDa. No accumulation of a protein in this molecular mass range was observed in extracellular or periplasmic fractions from the mutants. The results suggested that exoprotease loss is not simply the result of an inability to export protease from the periplasm because of outer membrane protein changes, as has been reported for certain mutants of some other gram-negative bacteria. Also, several growth conditions were used, including some that have been reported to influence outer membrane protein expression and permeability in other enteric gram-negative bacteria. Although exoprotease expression in A. salmonicida was influenced by these conditions, no major outer membrane protein changes which would correspond to changes observed in the mutants were observed in parent strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Prody C., Kustu S. Simple, rapid, and quantitative release of periplasmic proteins by chloroform. J Bacteriol. 1984 Dec;160(3):1181–1183. doi: 10.1128/jb.160.3.1181-1183.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus B. L., Carey A. M., Caron D. A., Kropinski A. M., Hancock R. E. Outer membrane permeability in Pseudomonas aeruginosa: comparison of a wild-type with an antibiotic-supersusceptible mutant. Antimicrob Agents Chemother. 1982 Feb;21(2):299–309. doi: 10.1128/aac.21.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Shaw D. H., Ishiguro E. E., Trust T. J. Structural and immunochemical homogeneity of Aeromonas salmonicida lipopolysaccharide. J Bacteriol. 1984 Apr;158(1):16–22. doi: 10.1128/jb.158.1.16-22.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darveau R. P., MacIntyre S., Buckley J. T., Hancock R. E. Purification and reconstitution in lipid bilayer membranes of an outer membrane, pore-forming protein of Aeromonas salmonicida. J Bacteriol. 1983 Dec;156(3):1006–1011. doi: 10.1128/jb.156.3.1006-1011.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J. S., Lallier R., Shaw D. H., Trust T. J. Electrophoretic and immunochemical analyses of the lipopolysaccharides from various strains of Aeromonas hydrophila. J Bacteriol. 1985 Oct;164(1):263–269. doi: 10.1128/jb.164.1.263-269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evenberg D., Versluis R., Lugtenberg B. Biochemical and immunological characterization of the cell surface of the fish pathogenic bacterium Aeromonas salmonicida. Biochim Biophys Acta. 1985 May 14;815(2):233–244. doi: 10.1016/0005-2736(85)90294-9. [DOI] [PubMed] [Google Scholar]
- Fecycz I. T., Campbell J. N. Mechanisms of activation and secretion of a cell-associated precursor of an exocellular protease of Pseudomonas aeruginosa 34362A. Eur J Biochem. 1985 Jan 2;146(1):35–42. doi: 10.1111/j.1432-1033.1985.tb08616.x. [DOI] [PubMed] [Google Scholar]
- Fyfe L., Coleman G., Munro A. L. Identification of major common extracellular proteins secreted by Aeromonas salmonicida strains isolated from diseased fish. Appl Environ Microbiol. 1987 Apr;53(4):722–726. doi: 10.1128/aem.53.4.722-726.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Harder K. J., Nikaido H., Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981 Oct;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Intracellular accumulation of extracellular proteins by pleiotropic export mutants of Aeromonas hydrophila. J Bacteriol. 1983 Apr;154(1):413–418. doi: 10.1128/jb.154.1.413-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtlein M., Schiessl S., Wagner W., Rdest U., Kreft J., Goebel W. Transport of hemolysin by Escherichia coli. J Cell Biochem. 1983;22(2):87–97. doi: 10.1002/jcb.240220203. [DOI] [PubMed] [Google Scholar]
- Ishiguro E. E., Kay W. W., Ainsworth T., Chamberlain J. B., Austen R. A., Buckley J. T., Trust T. J. Loss of virulence during culture of Aeromonas salmonicida at high temperature. J Bacteriol. 1981 Oct;148(1):333–340. doi: 10.1128/jb.148.1.333-340.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen S. E., Phillippe L., Teng Tseng J., Stemke G. W., Campbell J. N. Purification and characterization of exocellular proteases produced by a clinical isolate and a laboratory strain of Pseudomonas aeruginosa. Can J Microbiol. 1980 Jan;26(1):77–86. doi: 10.1139/m80-012. [DOI] [PubMed] [Google Scholar]
- Kay W. W., Buckley J. T., Ishiguro E. E., Phipps B. M., Monette J. P., Trust T. J. Purification and disposition of a surface protein associated with virulence of Aeromonas salmonicida. J Bacteriol. 1981 Sep;147(3):1077–1084. doi: 10.1128/jb.147.3.1077-1084.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay W. W., Phipps B. M., Ishiguro E. E., Olafson R. W., Trust T. J. Surface layer virulence A-proteins from Aeromonas salmonicida strains. Can J Biochem Cell Biol. 1984 Nov;62(11):1064–1071. doi: 10.1139/o84-137. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MacIntyre S., Trust T. J., Buckley J. T. Identification and characterization of outer membrane fragments released by Aeromonas sp. Can J Biochem. 1980 Oct;58(10):1018–1025. doi: 10.1139/o80-138. [DOI] [PubMed] [Google Scholar]
- McCashion R. N., Lynch W. H. Effects of polymyxin B nonapeptide on Aeromonas salmonicida. Antimicrob Agents Chemother. 1987 Sep;31(9):1414–1419. doi: 10.1128/aac.31.9.1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakae T. Outer-membrane permeability of bacteria. Crit Rev Microbiol. 1986;13(1):1–62. doi: 10.3109/10408418609108734. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai D. K. Significance of Extracellular Protease for Growth of a Heterotrophic Bacterium, Aeromonas salmonicida. Appl Environ Microbiol. 1985 Oct;50(4):1031–1037. doi: 10.1128/aem.50.4.1031-1037.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titball R. W., Bell A., Munn C. B. Role of caseinase from Aeromonas salmonicida in activation of hemolysin. Infect Immun. 1985 Sep;49(3):756–759. doi: 10.1128/iai.49.3.756-759.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titball R. W., Munn C. B. The purification and some properties of H-lysin from Aeromonas salmonicida. J Gen Microbiol. 1985 Jul;131(7):1603–1609. doi: 10.1099/00221287-131-7-1603. [DOI] [PubMed] [Google Scholar]
- Wood S. C., McCashion R. N., Lynch W. H. Multiple low-level antibiotic resistance in Aeromonas salmonicida. Antimicrob Agents Chemother. 1986 Jun;29(6):992–996. doi: 10.1128/aac.29.6.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]