Abstract
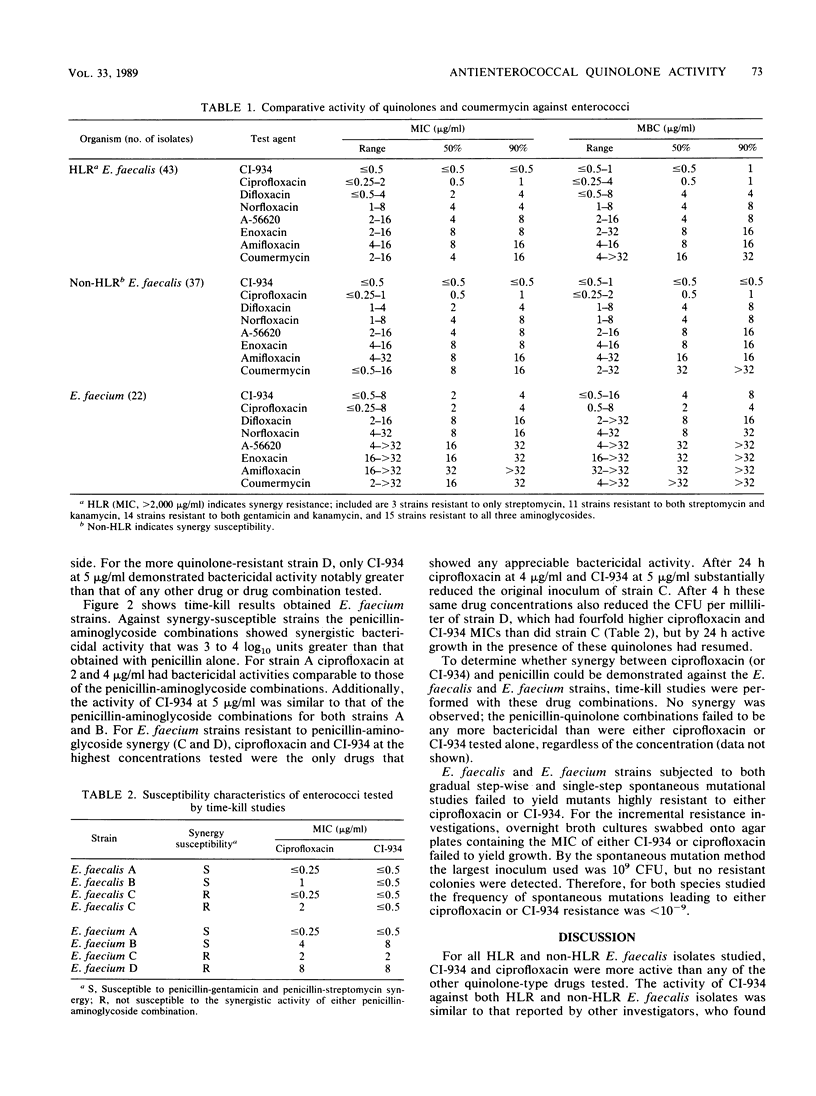
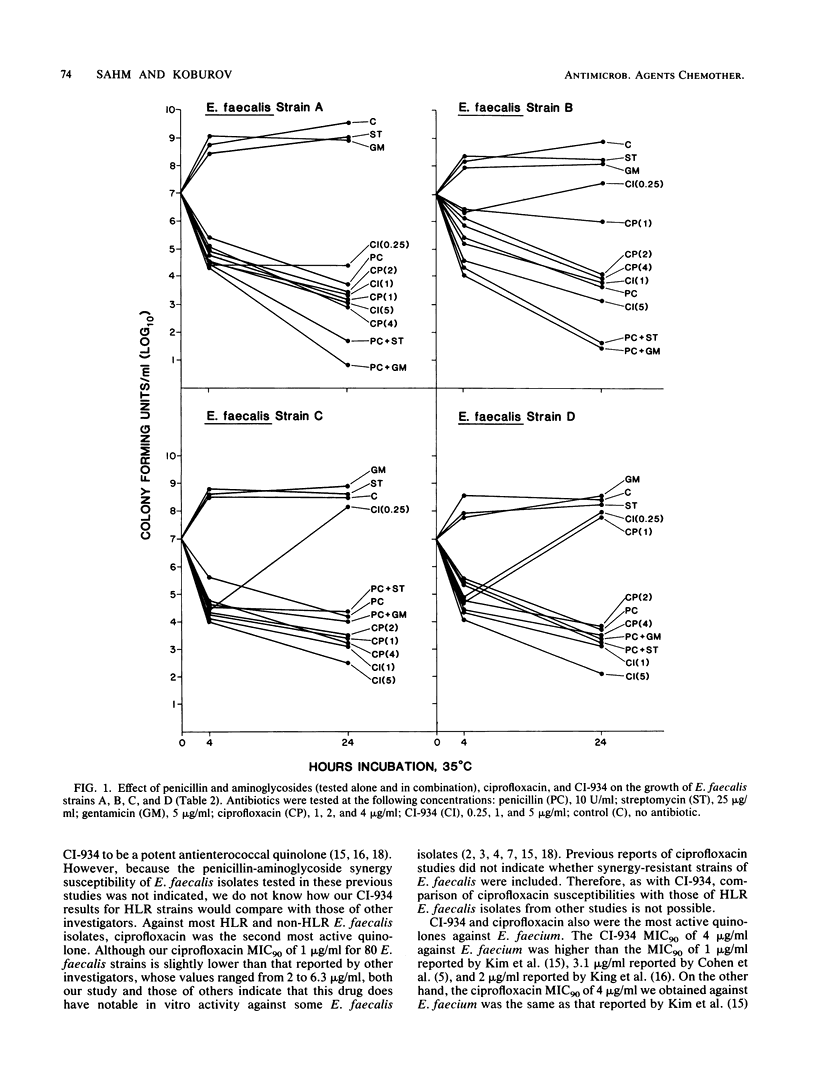
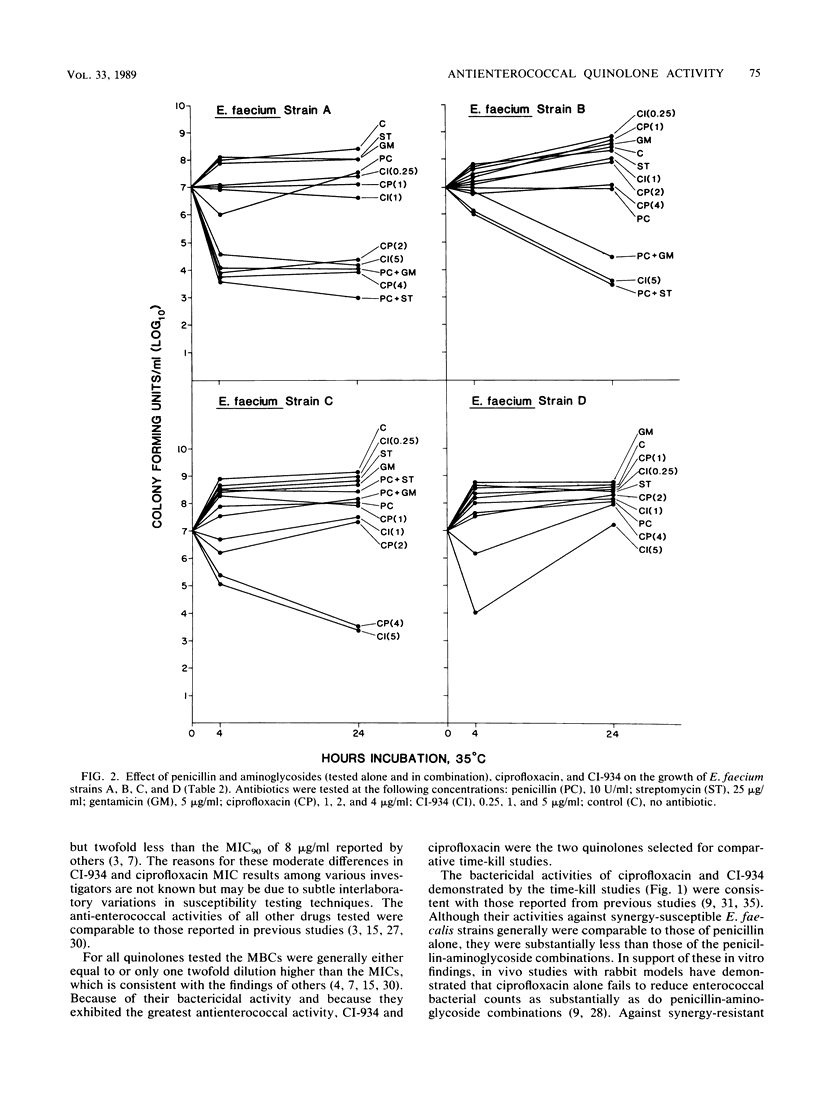
The MICs and MBCs of CI-934, ciprofloxacin, difloxacin (A-56619), A-56620, norfloxacin, enoxacin, amifloxacin, and coumermycin were determined for 43 clinical isolates of Enterococcus faecalis known to be resistant to penicillin-aminoglycoside synergy. Results were compared with those obtained for 37 synergy-susceptible E. faecalis and 22 Enterococcus faecium strains. Although no substantial differences in quinolone activities were observed between synergy-resistant and -susceptible E. faecalis strains, CI-934 and ciprofloxacin were the drugs that demonstrated the greatest bactericidal activity against both types of E. faecalis. The MBCs of the other quinolones were generally within a single twofold dilution of the MICs, but their antienterococcal activity did not approach that of CI-934 or ciprofloxacin. The MBCs for 90% of the isolates of CI-934 for synergy-resistant and -susceptible E. faecalis strains were 1 and less than or equal to 0.5 microgram/ml, respectively. The ciprofloxacin MBC for 90% of the E. faecalis strains tested was 1 microgram/ml. For E. faecium isolates the CI-934 and ciprofloxacin MBCs for 90% of the isolates were 8 and 4 micrograms/ml, respectively. Time-kill assays performed with synergy-susceptible enterococcal strains showed that the bactericidal activities of both CI-934 and ciprofloxacin were less than those of the penicillin-aminoglycoside combinations tested. However, against synergy-resistant isolates the activities of these two quinolones were comparable with and sometimes greater than those of penicillin-aminoglycoside combinations.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augé B., Avril J. L. Streptococcus faecalis: sensibilité aux quinolones, aux pénicillines et aux aminosides seuls ou associés. Pathol Biol (Paris) 1986 Nov;34(9):993–996. [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Thornsberry C., Ayers L. W., Gerlach E. H., Sommers H. M. Antibacterial activities of ciprofloxacin, norfloxacin, oxolinic acid, cinoxacin, and nalidixic acid. Antimicrob Agents Chemother. 1984 May;25(5):633–637. doi: 10.1128/aac.25.5.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Thornsberry C., Jones R. N. In vitro evaluation of A-56619 and A-56620, two new quinolones. Antimicrob Agents Chemother. 1986 Jan;29(1):40–43. doi: 10.1128/aac.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Ciprofloxacin, a quinolone carboxylic acid compound active against aerobic and anaerobic bacteria. Antimicrob Agents Chemother. 1984 Mar;25(3):319–326. doi: 10.1128/aac.25.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M. A., Griffin T. J., Bien P. A., Heifetz C. L., Domagala J. M. In vitro activity of CI-934, a quinolone carboxylic acid active against gram-positive and -negative bacteria. Antimicrob Agents Chemother. 1985 Dec;28(6):766–772. doi: 10.1128/aac.28.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P., Carlier C., Collatz E. Plasmid-mediated resistance to aminocyclitol antibiotics in group D streptococci. J Bacteriol. 1980 Aug;143(2):541–551. doi: 10.1128/jb.143.2.541-551.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Gardella A., Moellering R. C., Jr In vitro activity of ciprofloxacin, a new carboxyquinoline antimicrobial agent. Antimicrob Agents Chemother. 1984 Mar;25(3):331–335. doi: 10.1128/aac.25.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guerrero M., Rouse M. S., Henry N. K., Geraci J. E., Wilson W. R. In vitro and in vivo activity of ciprofloxacin against enterococci isolated from patients with infective endocarditis. Antimicrob Agents Chemother. 1987 Mar;31(3):430–433. doi: 10.1128/aac.31.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann S. A., Moellering R. C., Jr The enterococcus: "putting the bug in our ears". Ann Intern Med. 1987 May;106(5):757–761. doi: 10.7326/0003-4819-106-5-757. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., McHugh G. L., Winters M. B., Swartz M. N. Effects of novobiocin, coumermycin A1, clorobiocin, and their analogs on Escherichia coli DNA gyrase and bacterial growth. Antimicrob Agents Chemother. 1982 Oct;22(4):662–671. doi: 10.1128/aac.22.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingerman M., Pitsakis P. G., Rosenberg A., Hessen M. T., Abrutyn E., Murray B. E., Levison M. E. beta-Lactamase production in experimental endocarditis due to aminoglycoside-resistant Streptococcus faecalis. J Infect Dis. 1987 Jun;155(6):1226–1232. doi: 10.1093/infdis/155.6.1226. [DOI] [PubMed] [Google Scholar]
- Kim M. J., Weiser M., Gottschall S., Randall E. L. Identification of Streptococcus faecalis and Streptococcus faecium and susceptibility studies with newly developed antimicrobial agents. J Clin Microbiol. 1987 May;25(5):787–790. doi: 10.1128/jcm.25.5.787-790.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A., Shannon K., Slegg J., Phillips I. The in-vitro activity of CI-934 compared with that of other new 4-quinolones and nalidixic acid. J Antimicrob Chemother. 1986 Aug;18(2):163–169. doi: 10.1093/jac/18.2.163. [DOI] [PubMed] [Google Scholar]
- Mandell G. L., Kaye D., Levison M. E., Hook E. W. Enterococcal endocarditis. An analysis of 38 patients observed at the New York Hospital-Cornell Medical Center. Arch Intern Med. 1970 Feb;125(2):258–264. doi: 10.1001/archinte.125.2.258. [DOI] [PubMed] [Google Scholar]
- Mandell W., Neu H. C. In vitro activity of CI-934, a new quinolone, compared with that of other quinolones and other antimicrobial agents. Antimicrob Agents Chemother. 1986 May;29(5):852–857. doi: 10.1128/aac.29.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Korzeniowski O. M., Sande M. A., Wennersten C. B. Species-specific resistance to antimocrobial synergism in Streptococcus faecium and Streptococcus faecalis. J Infect Dis. 1979 Aug;140(2):203–208. doi: 10.1093/infdis/140.2.203. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Medrek T., Weinberg A. N. Prevalence of high-level resistance to aminoglycosides in clinical isolates of enterococci. Antimicrob Agents Chemother (Bethesda) 1970;10:335–340. [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinberg A. N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971 May;77(5):821–828. [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinstein A. J. Penicillin-tobramycin synergism against enterococci: a comparison with penicillin and gentamicin. Antimicrob Agents Chemother. 1973 Apr;3(4):526–529. doi: 10.1128/aac.3.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody J. A., Peterson L. R., Gerding D. N. In vitro activity of ciprofloxacin combined with azlocillin. Antimicrob Agents Chemother. 1985 Dec;28(6):849–850. doi: 10.1128/aac.28.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Mederski-Samaroj B. Transferable beta-lactamase. A new mechanism for in vitro penicillin resistance in Streptococcus faecalis. J Clin Invest. 1983 Sep;72(3):1168–1171. doi: 10.1172/JCI111042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Labthavikul P. Antibacterial activity of coumermycin alone and in combination with other antibiotics. Antimicrob Agents Chemother. 1984 Jun;25(6):687–689. doi: 10.1128/aac.25.6.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson L. R., Moody J. A., Fasching C. E., Gerding D. N. In vivo and in vitro activity of ciprofloxacin plus azlocillin against 12 streptococcal isolates in a neutropenic site model. Diagn Microbiol Infect Dis. 1987 Jun;7(2):127–136. doi: 10.1016/0732-8893(87)90030-7. [DOI] [PubMed] [Google Scholar]
- Sahm D. F., Torres C. Effects of medium and inoculum variations on screening for high-level aminoglycoside resistance in Enterococcus faecalis. J Clin Microbiol. 1988 Feb;26(2):250–256. doi: 10.1128/jcm.26.2.250-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm J. M., Hanson C. W., Chu D. T., Bailer R., Vojtko C., Fernandes P. B. In vitro evaluation of A-56619 (difloxacin) and A-56620: new aryl-fluoroquinolones. Antimicrob Agents Chemother. 1986 Feb;29(2):193–200. doi: 10.1128/aac.29.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton C. W., Liu C., Ratner H. B., Weeks L. S. Bactericidal activity of deptomycin (LY146032) compared with those of ciprofloxacin, vancomycin, and ampicillin against enterococci as determined by kill-kinetic studies. Antimicrob Agents Chemother. 1987 Jul;31(7):1014–1016. doi: 10.1128/aac.31.7.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenney J. H., Maack R. W., Chippendale G. R. Rapid selection of organisms with increasing resistance on subinhibitory concentrations of norfloxacin in agar. Antimicrob Agents Chemother. 1983 Jan;23(1):188–189. doi: 10.1128/aac.23.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger A. R., Murray B. E. Activity of LY146032 against Enterococci with and without high-level aminoglycoside resistance, including two penicillinase-producing strains. Antimicrob Agents Chemother. 1987 Nov;31(11):1779–1781. doi: 10.1128/aac.31.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourassowsky E., Van der Linden M. P., Lismont M. J., Crokaert F., Glupczynski Y. Rate of bactericidal activity for Streptococcus faecalis of a new quinolone, CI-934, compared with that of amoxicillin. Antimicrob Agents Chemother. 1986 Aug;30(2):258–259. doi: 10.1128/aac.30.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zervos M. J., Bacon A. E., 3rd, Patterson J. E., Schaberg D. R., Kauffman C. A. Enterococcal superinfection in patients treated with ciprofloxacin. J Antimicrob Chemother. 1988 Jan;21(1):113–115. doi: 10.1093/jac/21.1.113. [DOI] [PubMed] [Google Scholar]
- Zervos M. J., Kauffman C. A., Therasse P. M., Bergman A. G., Mikesell T. S., Schaberg D. R. Nosocomial infection by gentamicin-resistant Streptococcus faecalis. An epidemiologic study. Ann Intern Med. 1987 May;106(5):687–691. doi: 10.7326/0003-4819-106-5-687. [DOI] [PubMed] [Google Scholar]


