Abstract
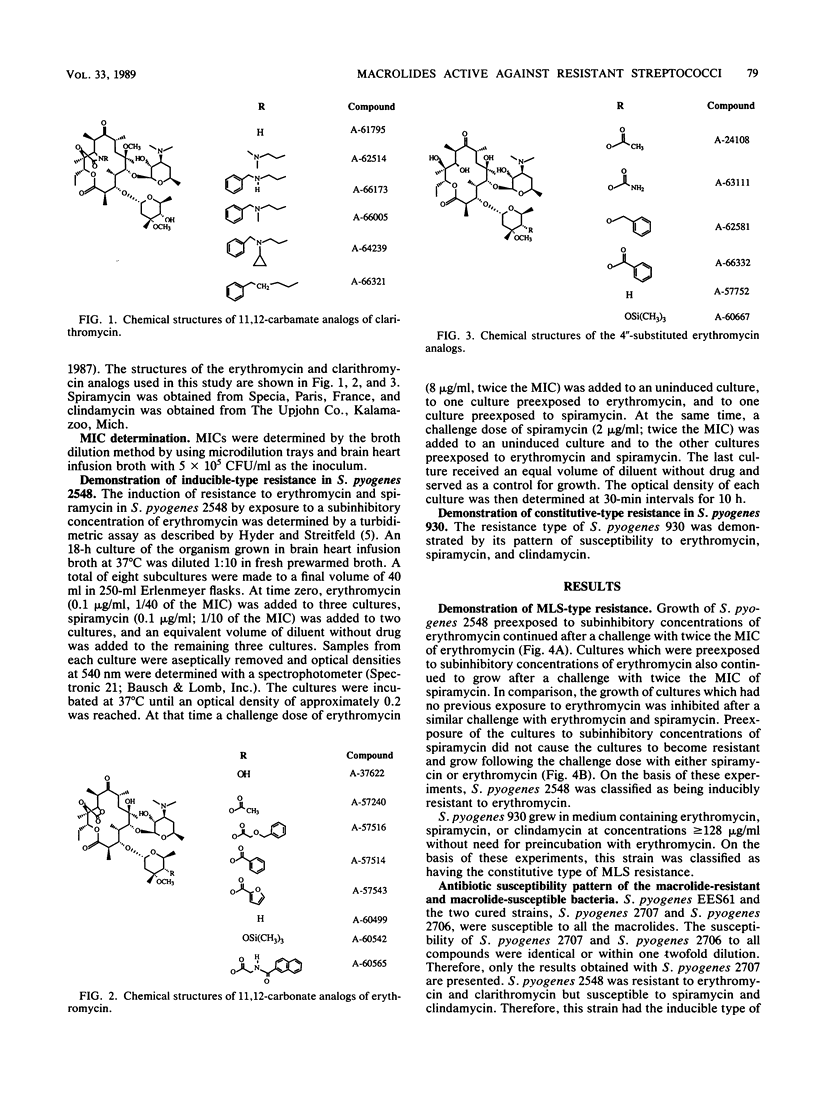
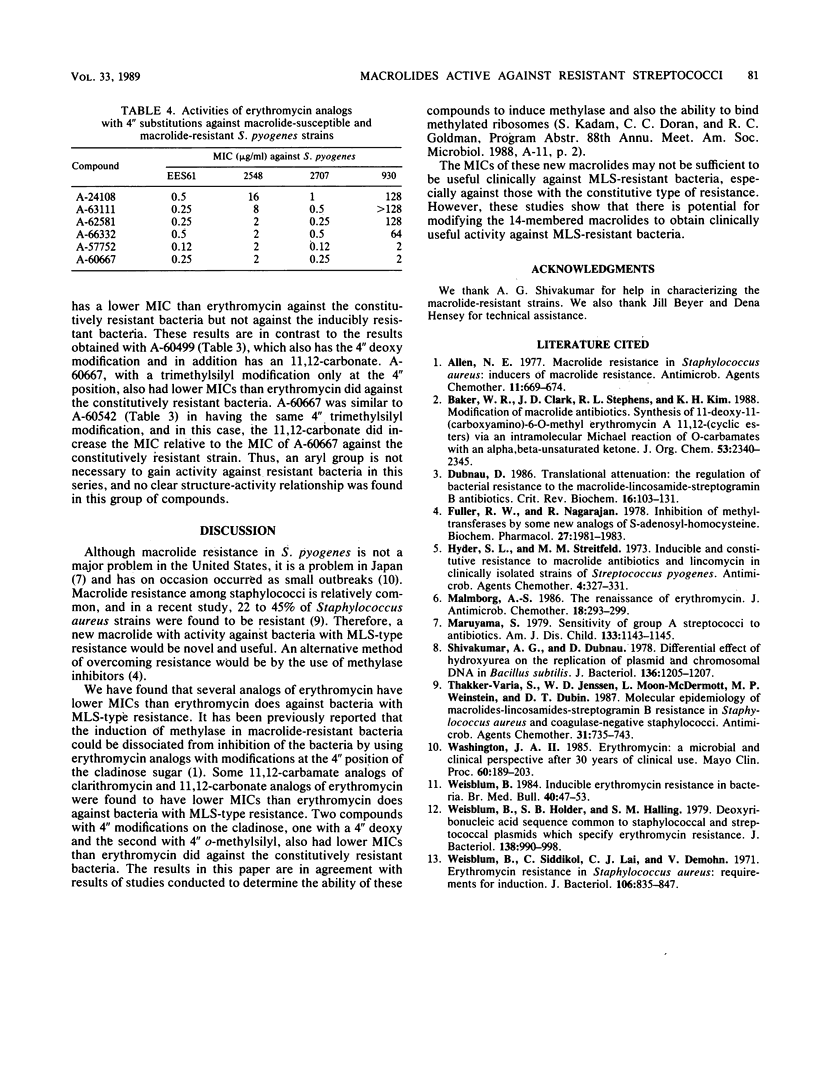
Macrolide-resistant bacteria can be classified as inducibly resistant or constitutively resistant. Inducibly resistant bacteria are resistant to 14-membered macrolides, such as erythromycin and clarithromycin (A-56268), but are susceptible to the 16-membered macrolides, such as tylosin and spiramycin, as well as to clindamycin. Constitutively resistant bacteria are resistant to macrolide-lincosamide-streptogramin B antibiotics. In this study, the MICs of several erythromycin and clarithromycin analogs against macrolide-susceptible and macrolide-resistant Streptococcus pyogenes strains were determined. Four 11,12-carbamate analogs of clarithromycin had lower MICs than erythromycin did against S. pyogenes with the inducible or constitutive type of macrolide-lincosamide-streptogramin B resistance. Five 11,12-carbonate analogs of erythromycin with modifications at the 4" position of cladinose had lower MICs than did erythromycin against S. pyogenes with the constitutive type of resistance, and one of these compounds, which had a naphthyl-glycyl substitution at the 4" position, had a lower MIC than erythromycin against both the inducibly resistant and constitutively resistant strains. Two analogs of erythromycin with a modification on the 4" position of cladinose had lower MICs than erythromycin did against the constitutively resistant organisms but not against the inducibly resistant organisms. Thus, 14-membered macrolides can be modified so as to confer a low MIC when tested in vitro.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E. Macrolide resistance in Staphylococcus aureus: inducers of macrolide resistance. Antimicrob Agents Chemother. 1977 Apr;11(4):669–674. doi: 10.1128/aac.11.4.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D. Translational attenuation: the regulation of bacterial resistance to the macrolide-lincosamide-streptogramin B antibiotics. CRC Crit Rev Biochem. 1984;16(2):103–132. doi: 10.3109/10409238409102300. [DOI] [PubMed] [Google Scholar]
- Fuller R. W., Nagarajan R. Inhibition of methyltransferases by some new analogs of S-adenosylhomocysteine. Biochem Pharmacol. 1978;27(15):1981–1983. doi: 10.1016/0006-2952(78)90018-7. [DOI] [PubMed] [Google Scholar]
- Hyder S. L., Streitfeld M. M. Inducible and constitutive resistance to macrolide antibiotics and lincomycin in clinically isolated strains of Streptococcus pyogenes. Antimicrob Agents Chemother. 1973 Sep;4(3):327–331. doi: 10.1128/aac.4.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmborg A. S. The renaissance of erythromycin. J Antimicrob Chemother. 1986 Sep;18(3):293–296. doi: 10.1093/jac/18.3.293. [DOI] [PubMed] [Google Scholar]
- Maruyama S., Yoshioka H., Fujita K., Takimoto M., Satake Y. Sensitivity of group A streptococci to antibiotics. Prevalence of resistance to erythromycin in Japan. Am J Dis Child. 1979 Nov;133(11):1143–1145. doi: 10.1001/archpedi.1979.02130110051007. [DOI] [PubMed] [Google Scholar]
- Shivakumar A. G., Dubnau D. Differential effect of hydroxyurea on the replication of plasmid and chromosomal DNA in Bacillus subtilis. J Bacteriol. 1978 Dec;136(3):1205–1207. doi: 10.1128/jb.136.3.1205-1207.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S., Jenssen W. D., Moon-McDermott L., Weinstein M. P., Dubin D. T. Molecular epidemiology of macrolides-lincosamides-streptogramin B resistance in Staphylococcus aureus and coagulase-negative staphylococci. Antimicrob Agents Chemother. 1987 May;31(5):735–743. doi: 10.1128/aac.31.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Wilson W. R. Erythromycin: a microbial and clinical perspective after 30 years of clinical use (1). Mayo Clin Proc. 1985 Mar;60(3):189–203. doi: 10.1016/s0025-6196(12)60219-5. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Holder S. B., Halling S. M. Deoxyribonucleic acid sequence common to staphylococcal and streptococcal plasmids which specify erythromycin resistance. J Bacteriol. 1979 Jun;138(3):990–998. doi: 10.1128/jb.138.3.990-998.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisblum B. Inducible erythromycin resistance in bacteria. Br Med Bull. 1984 Jan;40(1):47–53. doi: 10.1093/oxfordjournals.bmb.a071947. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Siddhikol C., Lai C. J., Demohn V. Erythromycin-inducible resistance in Staphylococcus aureus: requirements for induction. J Bacteriol. 1971 Jun;106(3):835–847. doi: 10.1128/jb.106.3.835-847.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


