Abstract
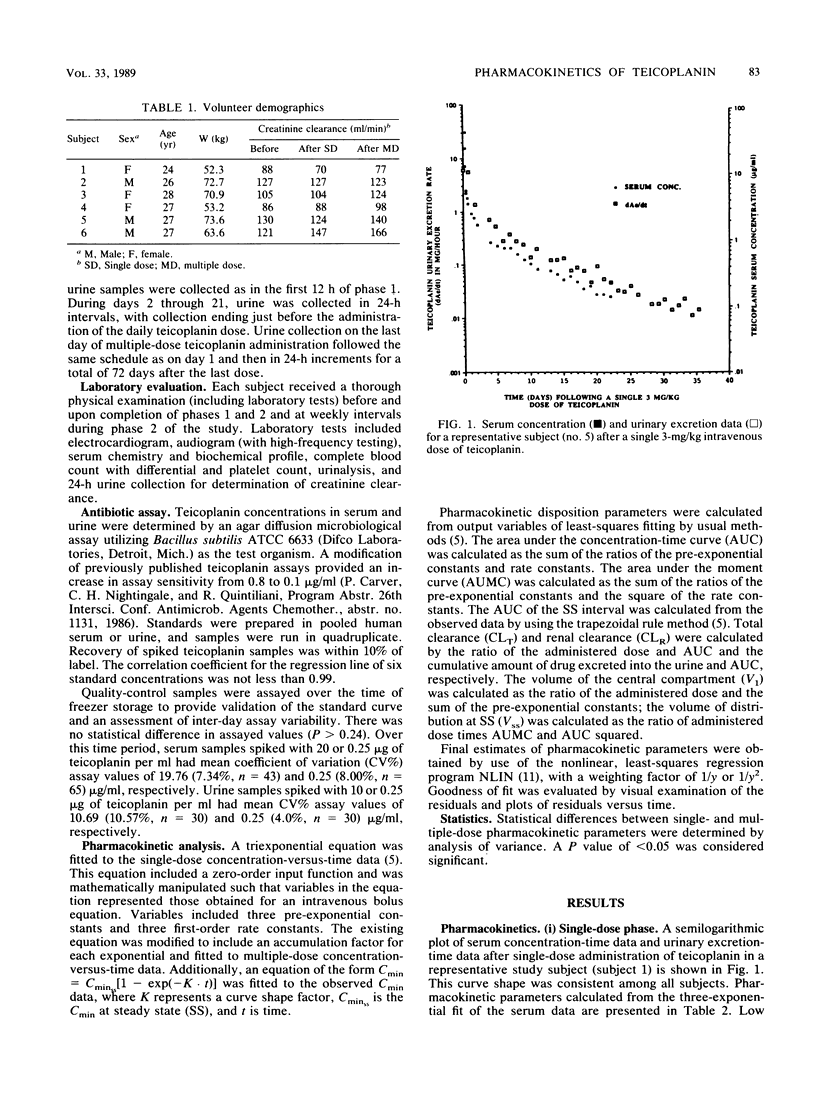
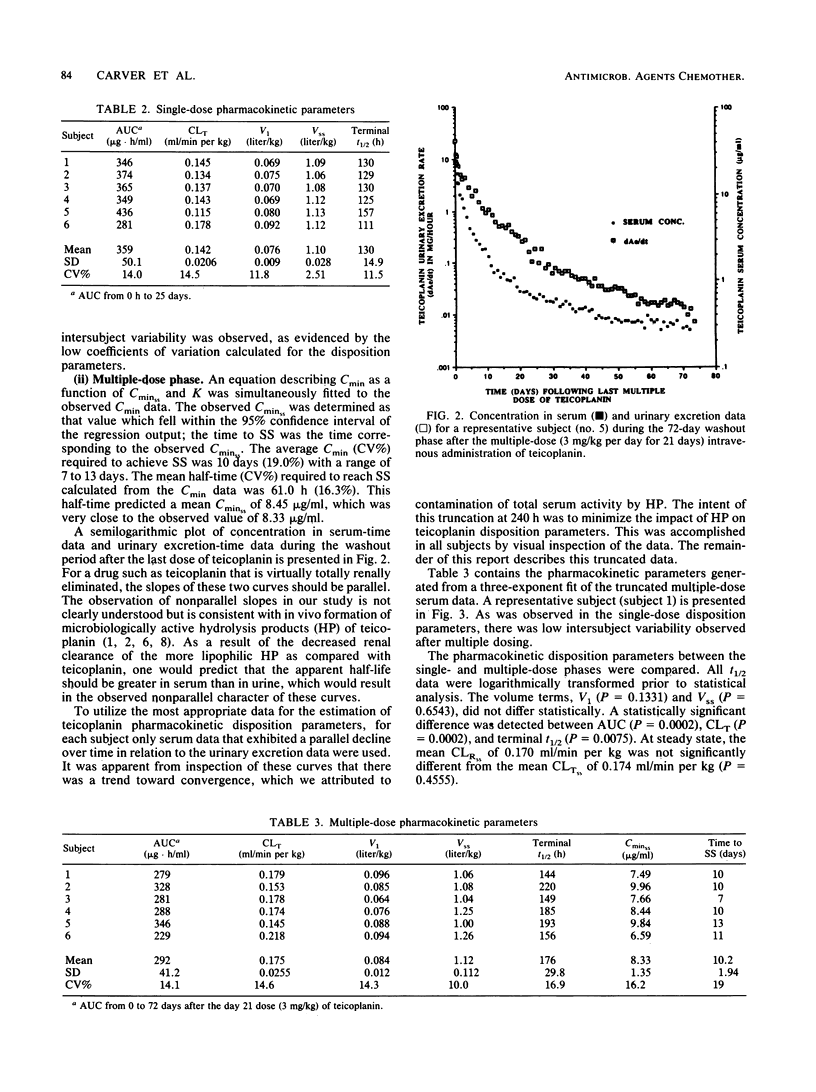
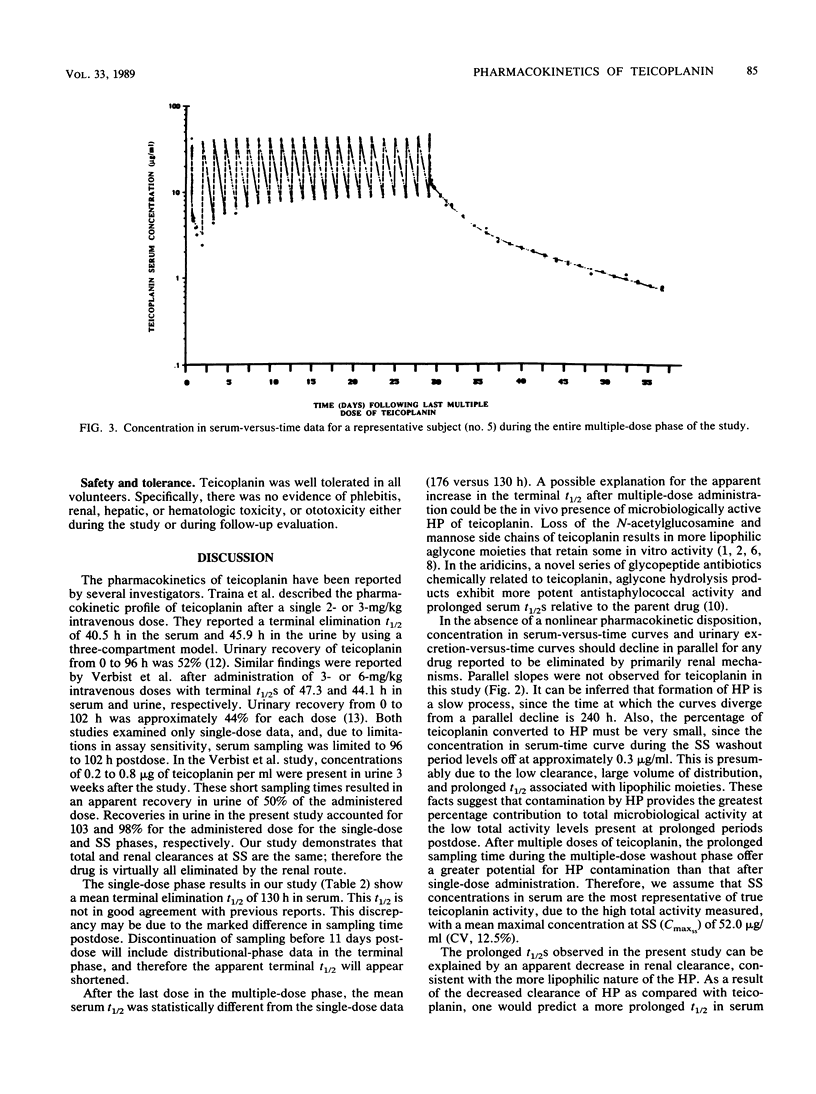
Teicoplanin, an investigational glycopeptide antibiotic related chemically and microbiologically to vancomycin, has in vitro and in vivo activity against gram-positive aerobic and anaerobic bacteria. We compared the single- and multiple-dose pharmacokinetics of intravenous teicoplanin in healthy volunteers. Serum and urine samples were collected for 35 days after single-dose (3 mg/kg) and 72 days after multiple-dose (3 mg/kg per day for 21 days) administration. A three-exponent equation with zero-order input was fitted to concentrations in serum. The mean half-lives (t1/2s) were significantly different (P = 0.0075) after single- and multiple-dose administration (130 +/- 14.9 and 176 +/- 29.8 h, respectively). The clinically relevant t1/2 obtained from multiple-dose data was approximately 61 h. Total and renal clearances determined at steady state were not statistically different, indicating that teicoplanin is eliminated almost entirely by renal mechanisms.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borghi A., Coronelli C., Faniuolo L., Allievi G., Pallanza R., Gallo G. G. Teichomycins, new antibiotics from Actinoplanes teichomyceticus nov. sp. IV. Separation and characterization of the components of teichomycin (teicoplanin). J Antibiot (Tokyo) 1984 Jun;37(6):615–620. doi: 10.7164/antibiotics.37.615. [DOI] [PubMed] [Google Scholar]
- Calain P., Krause K. H., Vaudaux P., Auckenthaler R., Lew D., Waldvogel F., Hirschel B. Early termination of a prospective, randomized trial comparing teicoplanin and flucloxacillin for treating severe staphylococcal infections. J Infect Dis. 1987 Feb;155(2):187–191. doi: 10.1093/infdis/155.2.187. [DOI] [PubMed] [Google Scholar]
- Craig W. A., Welling P. G. Protein binding of antimicrobials: clinical pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1977 Jul-Aug;2(4):252–268. doi: 10.2165/00003088-197702040-00002. [DOI] [PubMed] [Google Scholar]
- Malabarba A., Strazzolini P., Depaoli A., Landi M., Berti M., Cavalleri B. Teicoplanin, antibiotics from Actinoplanes teichomyceticus nov. sp. VI. Chemical degradation: physico-chemical and biological properties of acid hydrolysis products. J Antibiot (Tokyo) 1984 Sep;37(9):988–999. doi: 10.7164/antibiotics.37.988. [DOI] [PubMed] [Google Scholar]
- Pallanza R., Berti M., Scotti R., Randisi E., Arioli V. A-16686, a new antibiotic from Actinoplanes. II. Biological properties. J Antibiot (Tokyo) 1984 Apr;37(4):318–324. doi: 10.7164/antibiotics.37.318. [DOI] [PubMed] [Google Scholar]
- Pitkin D. H., Mico B. A., Sitrin R. D., Nisbet L. J. Charge and lipophilicity govern the pharmacokinetics of glycopeptide antibiotics. Antimicrob Agents Chemother. 1986 Mar;29(3):440–444. doi: 10.1128/aac.29.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traina G. L., Bonati M. Pharmacokinetics of teicoplanin in man after intravenous administration. J Pharmacokinet Biopharm. 1984 Apr;12(2):119–128. doi: 10.1007/BF01059273. [DOI] [PubMed] [Google Scholar]
- Verbist L., Tjandramaga B., Hendrickx B., Van Hecken A., Van Melle P., Verbesselt R., Verhaegen J., De Schepper P. J. In vitro activity and human pharmacokinetics of teicoplanin. Antimicrob Agents Chemother. 1984 Dec;26(6):881–886. doi: 10.1128/aac.26.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]


