Abstract
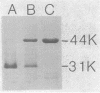
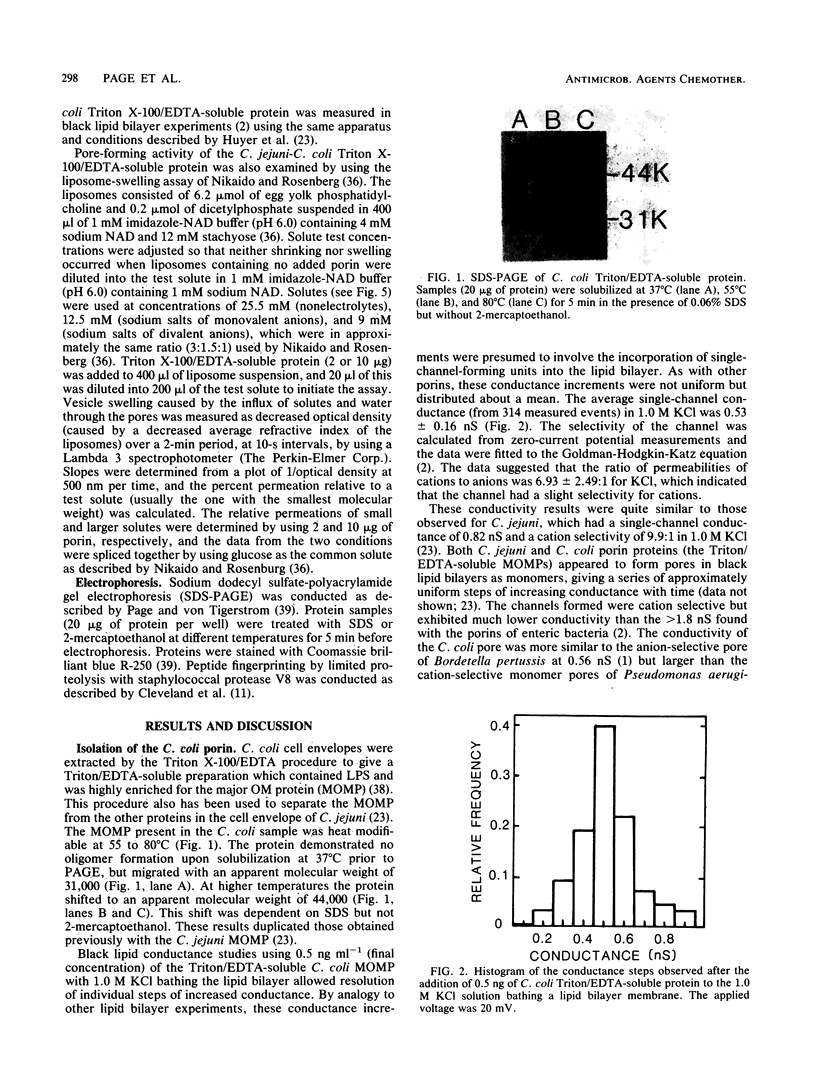
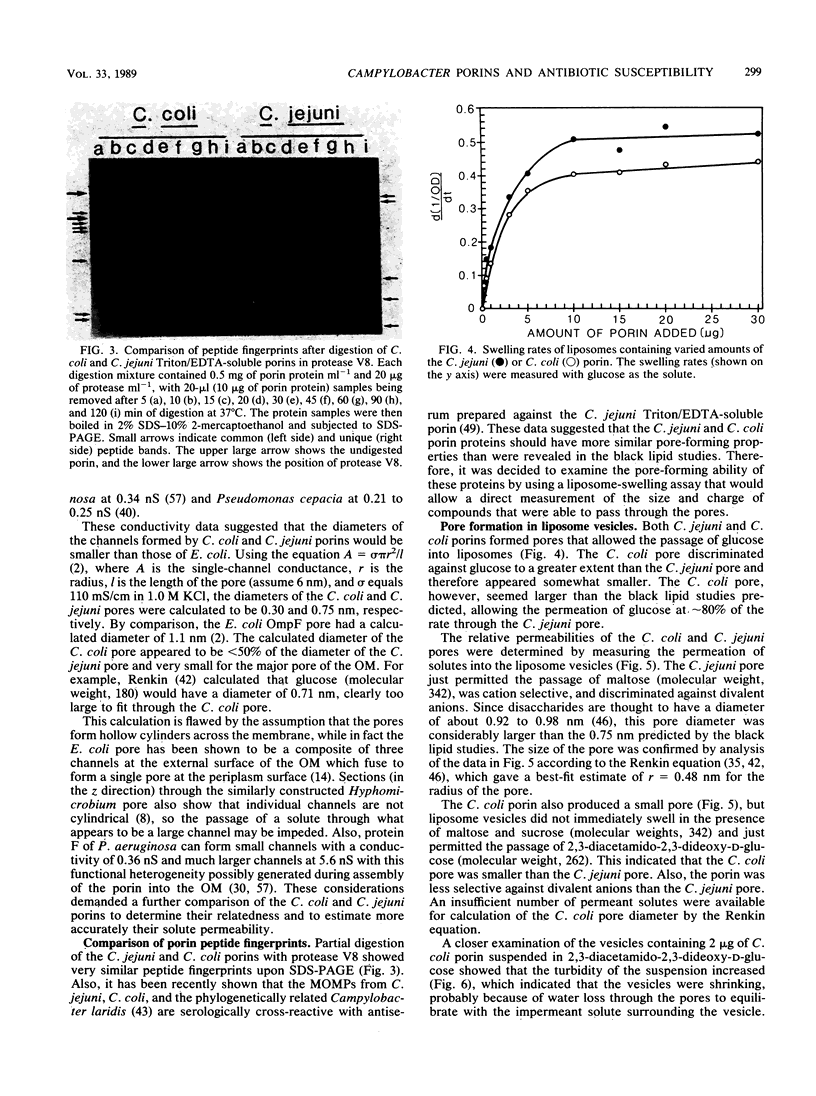
The major outer membrane protein was extracted from Campylobacter coli by Triton X-100/EDTA fractionation of cell envelopes. This heat-modifiable protein was shown to have pore-forming activity in black lipid bilayers. The C. coli porin formed a relatively small cation-selective pore with a mean single-channel conductance of 0.53 +/- 0.16 nS in 1.0 M KCl. There was no evidence of oligomer formation, which suggested that each protein monomer formed a pore. Pore-forming activity of the C. coli porin and similarly prepared Campylobacter jejuni porin was also measured in liposome-swelling assays. These results confirmed the cation selectivity of both pores. The C. coli porin formed a small pore, which hindered the penetration of solutes with a molecular weight of 262, and a larger pore, which hindered the penetration of solutes with a molecular weight of 340, in a protein-concentration-dependent manner. C. jejuni formed one size of pore that was slightly larger than the C. coli pore and just permitted the passage of solutes, with a molecular weight of 340. A review of the literature concerning in vitro screening of antimicrobial agents tended to confirm the low permeability of the C. jejuni outer membrane to hydrophilic antimicrobial agents except when the molecules had molecular weights of less than 360. The porins of C. jejuni and C. coli may contribute to intrinsic resistance to antimicrobial agents, whereas alternative (nonporin) routes of antimicrobial agent uptake may be more important determinants of susceptibility to antimicrobial agents.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong S. K., Parr T. R., Jr, Parker C. D., Hancock R. E. Bordetella pertussis major outer membrane porin protein forms small, anion-selective channels in lipid bilayer membranes. J Bacteriol. 1986 Apr;166(1):212–216. doi: 10.1128/jb.166.1.212-216.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz R., Schmid A., Hancock R. E. Ion selectivity of gram-negative bacterial porins. J Bacteriol. 1985 May;162(2):722–727. doi: 10.1128/jb.162.2.722-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buck G. E., Kelly M. T. Susceptibility testing of Campylobacter fetus subsp. jejuni, using broth microdilution panels. Antimicrob Agents Chemother. 1982 Feb;21(2):274–277. doi: 10.1128/aac.21.2.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge R., Warren C., Phillips I. Macrolide, lincosamide and streptogramin resistance in Campylobacter jejuni/coli. J Antimicrob Chemother. 1986 Mar;17(3):315–321. doi: 10.1093/jac/17.3.315. [DOI] [PubMed] [Google Scholar]
- Butzler J. P., Skirrow M. B. Campylobacter enteritis. Clin Gastroenterol. 1979 Sep;8(3):737–765. [PubMed] [Google Scholar]
- Chalcroft J. P., Engelhardt H., Baumeister W. Structure of the porin from a bacterial stalk. FEBS Lett. 1987 Jan 19;211(1):53–58. doi: 10.1016/0014-5793(87)81273-5. [DOI] [PubMed] [Google Scholar]
- Chartrand S. A., Scribner R. K., Weber A. H., Welch D. F., Marks M. I. In vitro activity of CI-919 (AT-2266), an oral antipseudomonal compound. Antimicrob Agents Chemother. 1983 May;23(5):658–663. doi: 10.1128/aac.23.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. In vitro activity of enoxacin, a quinolone carboxylic acid, compared with those of norfloxacin, new beta-lactams, aminoglycosides, and trimethoprim. Antimicrob Agents Chemother. 1983 Nov;24(5):754–763. doi: 10.1128/aac.24.5.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Della Bruna C., Schioppacassi G., Ungheri D., Jabès D., Morvillo E., Sanfilippo A. LM 427, a new spiropiperidylrifamycin: in vitro and in vivo studies. J Antibiot (Tokyo) 1983 Nov;36(11):1502–1506. doi: 10.7164/antibiotics.36.1502. [DOI] [PubMed] [Google Scholar]
- Elharrif Z., Mégraud F., Marchand A. M. Susceptibility of Campylobacter jejuni and Campylobacter coli to macrolides and related compounds. Antimicrob Agents Chemother. 1985 Nov;28(5):695–697. doi: 10.1128/aac.28.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A., Massalski A., Schindler H., Dorset D. L., Rosenbusch J. P. Porin channel triplets merge into single outlets in Escherichia coli outer membranes. Nature. 1985 Oct 17;317(6038):643–645. doi: 10.1038/317643a0. [DOI] [PubMed] [Google Scholar]
- Fliegelman R. M., Petrak R. M., Goodman L. J., Segreti J., Trenholme G. M., Kaplan R. L. Comparative in vitro activities of twelve antimicrobial agents against Campylobacter species. Antimicrob Agents Chemother. 1985 Mar;27(3):429–430. doi: 10.1128/aac.27.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudreau C. L., Lariviere L. A., Lauzer J. C., Turgeon F. F. Effect of clavulanic acid on susceptibility of Campylobacter jejuni and Campylobacter coli to eight beta-lactam antibiotics. Antimicrob Agents Chemother. 1987 Jun;31(6):940–942. doi: 10.1128/aac.31.6.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman L. J., Fliegelman R. M., Trenholme G. M., Kaplan R. L. Comparative in vitro activity of ciprofloxacin against Campylobacter spp. and other bacterial enteric pathogens. Antimicrob Agents Chemother. 1984 Apr;25(4):504–506. doi: 10.1128/aac.25.4.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens H., De Mol P., Coignau H., Levy J., Grados O., Ghysels G., Innocent H., Butzler J. P. Comparative in vitro activities of aztreonam, ciprofloxacin, norfloxacin, ofloxacin, HR 810 (a new cephalosporin), RU28965 (a new macrolide), and other agents against enteropathogens. Antimicrob Agents Chemother. 1985 Mar;27(3):388–392. doi: 10.1128/aac.27.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Hancock R. E., Decad G. M., Nikaido H. Identification of the protein producing transmembrane diffusion pores in the outer membrane of Pseudomonas aeruginosa PA01. Biochim Biophys Acta. 1979 Jul 5;554(2):323–331. doi: 10.1016/0005-2736(79)90373-0. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Hosaka M., Oomori Y., Niwata Y., Suzue S., Irikura T. In vitro and in vivo antibacterial activity of AM-833, a new quinolone derivative. Antimicrob Agents Chemother. 1986 Jun;29(6):1059–1066. doi: 10.1128/aac.29.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof H., Rieffert M., Sticht-Groh V., Zak O., Schweizer E. H. Antibacterial activities of nitrothiazole against Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1984 Oct;26(4):498–500. doi: 10.1128/aac.26.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., De Grandis S., Fleming P. C. Antimicrobial susceptibility of Campylobacter jejuni with special reference to resistance patterns of Canadian isolates. Antimicrob Agents Chemother. 1981 Apr;19(4):593–597. doi: 10.1128/aac.19.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotarski S. F., Merriwether T. L., Tkalcevic G. T., Gemski P. Genetic studies of kanamycin resistance in Campylobacter jejuni. Antimicrob Agents Chemother. 1986 Aug;30(2):225–230. doi: 10.1128/aac.30.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariviere L. A., Gaudreau C. L., Turgeon F. F. Susceptibility of clinical isolates of Campylobacter jejuni to twenty-five antimicrobial agents. J Antimicrob Chemother. 1986 Dec;18(6):681–685. doi: 10.1093/jac/18.6.681. [DOI] [PubMed] [Google Scholar]
- Leive L., Telesetsky S., Coleman W. G., Jr, Carr D. Tetracyclines of various hydrophobicities as a probe for permeability of Escherichia coli outer membranes. Antimicrob Agents Chemother. 1984 May;25(5):539–544. doi: 10.1128/aac.25.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan S. M., Trust T. J. Structural and antigenic heterogeneity of lipopolysaccharides of Campylobacter jejuni and Campylobacter coli. Infect Immun. 1984 Jul;45(1):210–216. doi: 10.1128/iai.45.1.210-216.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel J., Rogol M., Dickman D. Susceptibility of clinical isolates of Campylobacter jejuni to sixteen antimicrobial agents. Antimicrob Agents Chemother. 1983 May;23(5):796–797. doi: 10.1128/aac.23.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. A., Woodruff W. A., Hancock R. E. Antibiotic uptake pathways across the outer membrane of Pseudomonas aeruginosa. Antibiot Chemother (1971) 1987;39:172–181. doi: 10.1159/000414344. [DOI] [PubMed] [Google Scholar]
- Muytjens H. L., van der Ros-van de Repe J. Comparative activities of 13 beta-lactam antibiotics. Antimicrob Agents Chemother. 1982 Jun;21(6):925–934. doi: 10.1128/aac.21.6.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Minami A., Katae H., Inoue S., Yamagishi J., Takase Y., Shimizu M. In vitro antibacterial properties of AT-2266, a new pyridonecarboxylic acid. Antimicrob Agents Chemother. 1983 May;23(5):641–648. doi: 10.1128/aac.23.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Role of permeability barriers in resistance to beta-lactam antibiotics. Pharmacol Ther. 1985;27(2):197–231. doi: 10.1016/0163-7258(85)90069-5. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Effect on solute size on diffusion rates through the transmembrane pores of the outer membrane of Escherichia coli. J Gen Physiol. 1981 Feb;77(2):121–135. doi: 10.1085/jgp.77.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y. Porin channels in Escherichia coli: studies with liposomes reconstituted from purified proteins. J Bacteriol. 1983 Jan;153(1):241–252. doi: 10.1128/jb.153.1.241-252.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido H., Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985 Mar;49(1):1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page W. J., Taylor D. E. Comparison of methods used to separate the inner and outer membranes of cell envelopes of Campylobacter spp. J Gen Microbiol. 1988 Nov;134(11):2925–2932. doi: 10.1099/00221287-134-11-2925. [DOI] [PubMed] [Google Scholar]
- Page W. J., von Tigerstrom M. Iron- and molybdenum-repressible outer membrane proteins in competent Azotobacter vinelandii. J Bacteriol. 1982 Jul;151(1):237–242. doi: 10.1128/jb.151.1.237-242.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr T. R., Jr, Moore R. A., Moore L. V., Hancock R. E. Role of porins in intrinsic antibiotic resistance of Pseudomonas cepacia. Antimicrob Agents Chemother. 1987 Jan;31(1):121–123. doi: 10.1128/aac.31.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez Perez G. I., Blaser M. J. Lipopolysaccharide characteristics of pathogenic campylobacters. Infect Immun. 1985 Feb;47(2):353–359. doi: 10.1128/iai.47.2.353-359.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Romaniuk P. J., Zoltowska B., Trust T. J., Lane D. J., Olsen G. J., Pace N. R., Stahl D. A. Campylobacter pylori, the spiral bacterium associated with human gastritis, is not a true Campylobacter sp. J Bacteriol. 1987 May;169(5):2137–2141. doi: 10.1128/jb.169.5.2137-2141.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULTZ S. G., SOLOMON A. K. Determination of the effective hydrodynamic radii of small molecules by viscometry. J Gen Physiol. 1961 Jul;44:1189–1199. doi: 10.1085/jgp.44.6.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagara H., Mochizuki A., Okamura N., Nakaya R. Antimicrobial resistance of Campylobacter jejuni and Campylobacter coli with special reference to plasmid profiles of Japanese clinical isolates. Antimicrob Agents Chemother. 1987 May;31(5):713–719. doi: 10.1128/aac.31.5.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- Shanker S., Sorrell T. C. Susceptibility of Campylobacter jejuni to twenty-three antimicrobial agents. Pathology. 1983 Jan;15(1):61–63. doi: 10.3109/00313028309061403. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Weinberg E., Gadebusch H. H. In vitro antibacterial activity of norfloxacin (MK-0366, AM-715) and other agents against gastrointestinal tract pathogens. Antimicrob Agents Chemother. 1983 Jan;23(1):86–90. doi: 10.1128/aac.23.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Chang N. Immunoblot and enzyme-linked immunosorbent assays of Campylobacter major outer-membrane protein and application to the differentiation of Campylobacter species. Mol Cell Probes. 1987 Sep;1(3):261–274. doi: 10.1016/0890-8508(87)90038-7. [DOI] [PubMed] [Google Scholar]
- Taylor D. E., Courvalin P. Mechanisms of antibiotic resistance in Campylobacter species. Antimicrob Agents Chemother. 1988 Aug;32(8):1107–1112. doi: 10.1128/aac.32.8.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. E., Garner R. S., Allan B. J. Characterization of tetracycline resistance plasmids from Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1983 Dec;24(6):930–935. doi: 10.1128/aac.24.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera P., Scorneaux B. In vitro susceptibility of Campylobacter jejuni to 27 antimicrobial agents and various combinations of beta-lactams with clavulanic acid or sulbactam. Antimicrob Agents Chemother. 1985 Jul;28(1):37–40. doi: 10.1128/aac.28.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoof R., Goossens H., Coignau H., Stas G., Butzler J. P. Susceptibility pattern of Campylobacter jejuni from human and animal origins to different antimicrobial agents. Antimicrob Agents Chemother. 1982 Jun;21(6):990–992. doi: 10.1128/aac.21.6.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhoof R., Vanderlinden M. P., Dierickx R., Lauwers S., Yourassowsky E., Butzler J. P. Susceptibility of Campylobacter fetus subsp. jejuni to twenty-nine antimicrobial agents. Antimicrob Agents Chemother. 1978 Oct;14(4):553–556. doi: 10.1128/aac.14.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder M. Susceptibility of Campylobacter fetus subsp. jejuni to twenty antimicrobiol agents. Antimicrob Agents Chemother. 1979 Jul;16(1):37–39. doi: 10.1128/aac.16.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. L., Reller L. B., Blaser M. J. Comparison of antimicrobial susceptibility patterns of Campylobacter jejuni and Campylobacter coli. Antimicrob Agents Chemother. 1984 Sep;26(3):351–353. doi: 10.1128/aac.26.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Parr T. R., Jr, Hancock R. E., Hanne L. F., Nicas T. I., Iglewski B. H. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986 Aug;167(2):473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]