Abstract
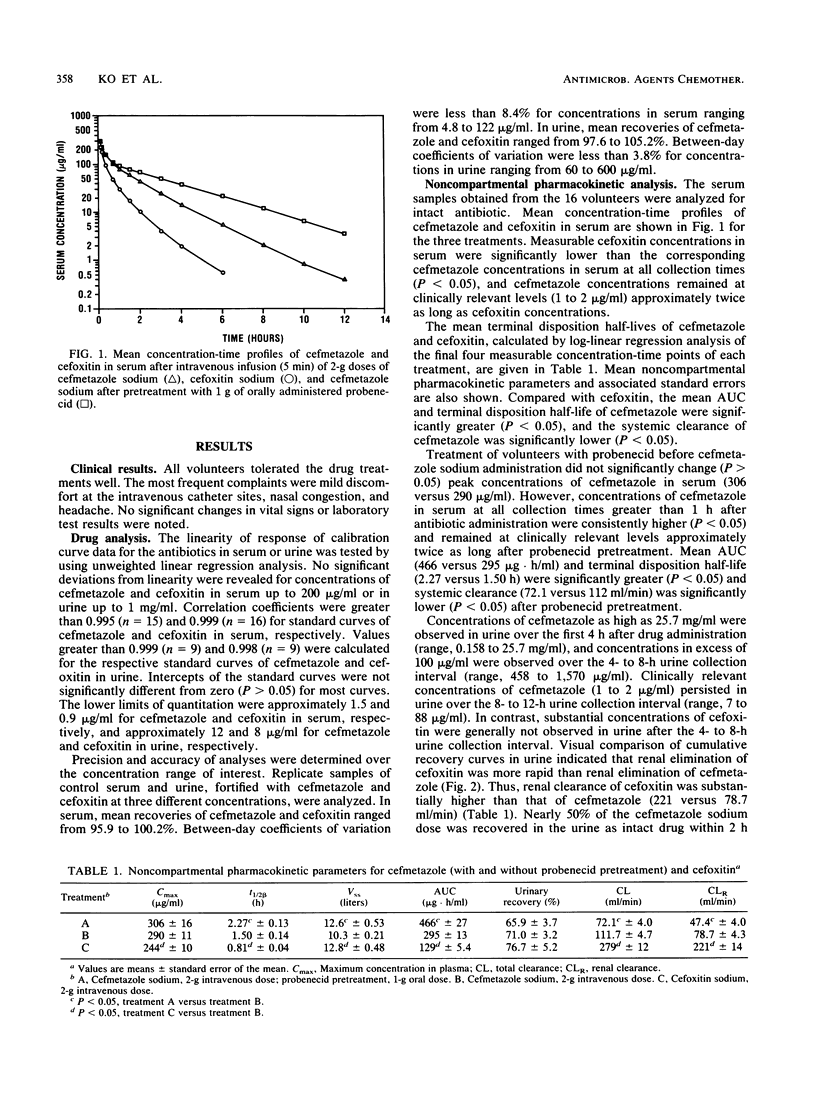
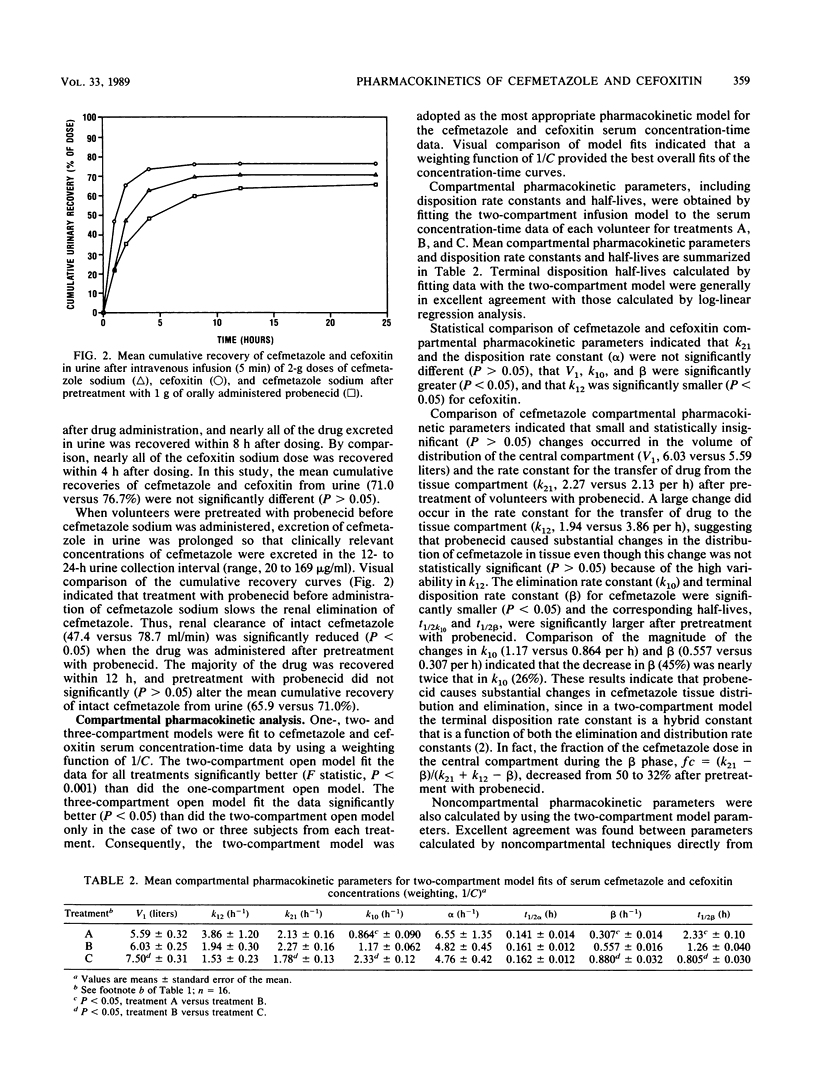
Sixteen healthy male volunteers participated in a randomized, balanced, three-way crossover study comparing the pharmacokinetics of cefmetazole, cefoxitin, and cefmetazole with probenecid pretreatment. Single 2-g doses of cefmetazole sodium and cefoxitin sodium were given intravenously as a 5-min infusion. Concentrations of cefmetazole and cefoxitin were determined by using a specific semiautomated high-performance liquid chromatographic method. Concentration-time profiles of cefmetazole and cefoxitin declined in a biexponential manner from peak levels. Compared with cefoxitin, cefmetazole had a significantly (P less than 0.05) higher mean (+/- standard error of the mean) peak concentration in serum (290 +/- 11 versus 244 +/- 10 micrograms/ml), a longer terminal disposition half-life (1.50 +/- 0.14 versus 0.81 +/- 0.04 h), lower systemic clearance (111.7 +/- 4.7 versus 279 +/- 12 ml/min) and renal clearance (78.7 +/- 4.3 versus 221 +/- 14 ml/min) of intact drug, and a slightly smaller steady-state volume of distribution (10.3 +/- 0.21 versus 12.8 +/- 0.48 liters). Mean recoveries of cefmetazole and cefoxitin in urine were approximately 71 and 77%, respectively. Pretreatment of volunteers with probenecid (1 g orally) significantly (P less than 0.05) increased concentrations of cefmetazole in serum 1 h after drug administration without significantly increasing maximum concentrations in serum. Mean areas under the concentration-time curve (466 +/- 27 versus 295 +/- 13 micrograms.h/ml) and terminal disposition half-lives (2.27 +/- 0.13 versus 1.50 +/- 0.14 h) of cefmetazole increased. Systemic clearance (72.1 +/- 4.0 versus 111.7 +/- 4.7 ml/min) and renal clearance (47.4 +/- 4.0 versus 78.7 +/- 4.3 ml/min) of intact antibiotic decreased. Mean recoveries (65.9 +/- 3.7 versus 71.0 +/- 3.2%) of intact cefmetazole in urine were not significantly (P > 0.05) different. Elimination of cefmetazole in urine was also significantly prolonged by probenecid, with substantial concentrations of cefmetazole (>/= 20 micrograms/ml) found in the 12- to 24-h urine collection for 14 to 16 volunteers. The results show that cefmetazole remains at clinically relevant concentrations (1 to 2 micrograms/ml) approximately twice as long as cefoxitin, that serum cefmetazole can be maintained longer at clinically significant concentrations with preadministration of probenecid, and that cefmetazole is partially eliminated by renal tubule secretion.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cunningham R. F., Israili Z. H., Dayton P. G. Clinical pharmacokinetics of probenecid. Clin Pharmacokinet. 1981 Mar-Apr;6(2):135–151. doi: 10.2165/00003088-198106020-00004. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Raftery E. B., Goldberg A. D., Skeggs H., Till A. E., Martin C. M. Effects of rate of infusion and probenecid on serum levels, renal excretion, and tolerance of intravenous doses of cefoxitin in humans: comparison with cephalothin. Antimicrob Agents Chemother. 1974 Sep;6(3):338–346. doi: 10.1128/aac.6.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L., Fuchs P. C., Thornsberry C. Antimicrobial activity of cefmetazole (CS-1170) and recommendations for susceptibility testing by disk diffusion, dilution, and anaerobic methods. J Clin Microbiol. 1986 Dec;24(6):1055–1059. doi: 10.1128/jcm.24.6.1055-1059.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao H., Yanagisawa H., Shimizu B., Kaneko M., Nagano M. A new semisynthetic 7alpha-methoxycephalosporin, CS-1170: 7beta-((cyanomethyl)thio)acetamido)-7alpha-methoxy-3-((1-methyl-1H-tetrazol-5-yl)thio)methyl)-3-cephem-4-carboxylic acid. J Antibiot (Tokyo) 1976 May;29(5):554–558. doi: 10.7164/antibiotics.29.554. [DOI] [PubMed] [Google Scholar]
- Ohkawa M., Orito M., Sugata T., Shimamura M., Sawaki M., Nakashita E., Kuroda K., Sasahara K. Pharmacokinetics of cefmetazole in normal subjects and in patients with impaired renal function. Antimicrob Agents Chemother. 1980 Sep;18(3):386–389. doi: 10.1128/aac.18.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barbero J., Mariño E. L., Dominguez-Gil A. Pharmacokinetics of cefmetazole administered intramuscularly and intravenously to healthy adults. Antimicrob Agents Chemother. 1985 Oct;28(4):544–547. doi: 10.1128/aac.28.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez J. R., Mariño E. L., Otero M. J., Commes J. R., Garcia J., Rodriguez J. M., Lozano F., Dominguez-Gil A., Alonso A. G. Concentrations of cefmetazole in plasma and tissue resulting from peri-incisional administration before appendectomy. Antimicrob Agents Chemother. 1984 Nov;26(5):787–788. doi: 10.1128/aac.26.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrogie J. J., Davies R. O., Yeh K. C., Rogers D., Holmes G. I., Skeggs H., Martin C. M. Bioavailability and pharmacokinetics of cefoxitin sodium. J Antimicrob Chemother. 1978 Jul;4(B):69–78. doi: 10.1093/jac/4.suppl_b.69. [DOI] [PubMed] [Google Scholar]
- Sonneville P. F., Kartodirdjo R. R., Skeggs H., Till A. E., Martin C. M. Comparative clinical pharmacology of intravenous cefoxitin and cephalothin. Eur J Clin Pharmacol. 1976 Mar 22;09(5-6):397–403. doi: 10.1007/BF00606555. [DOI] [PubMed] [Google Scholar]


