Abstract
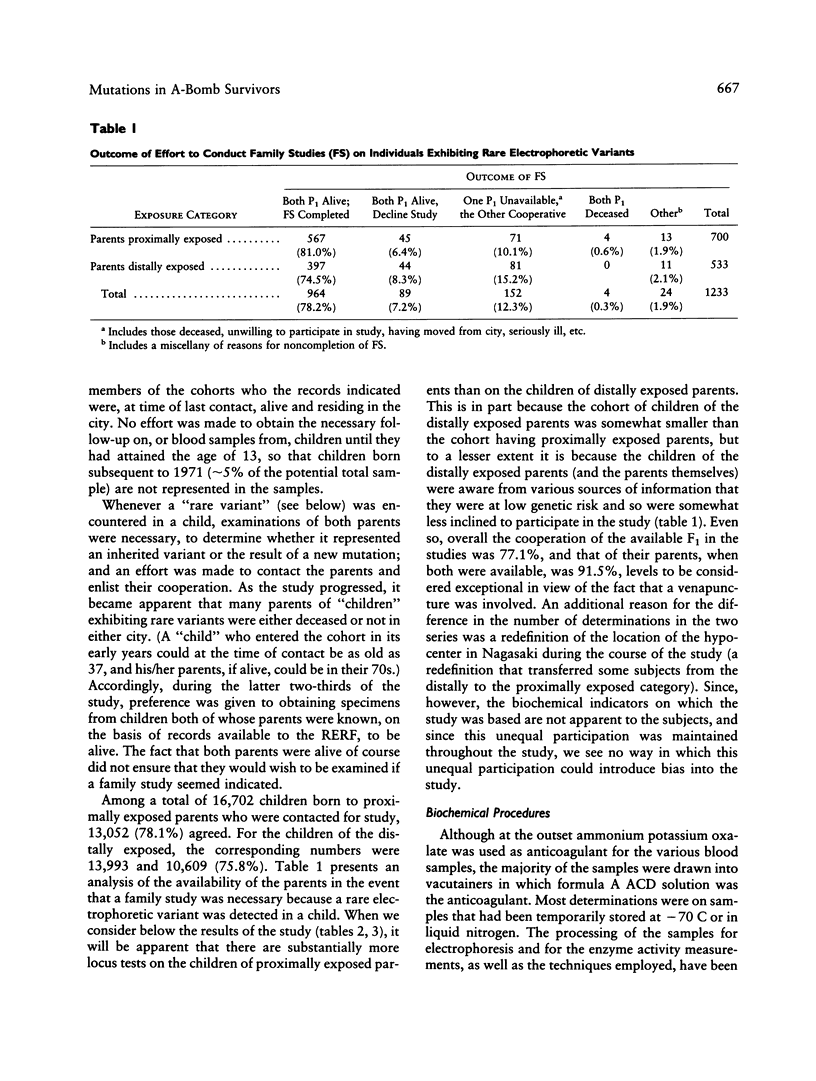
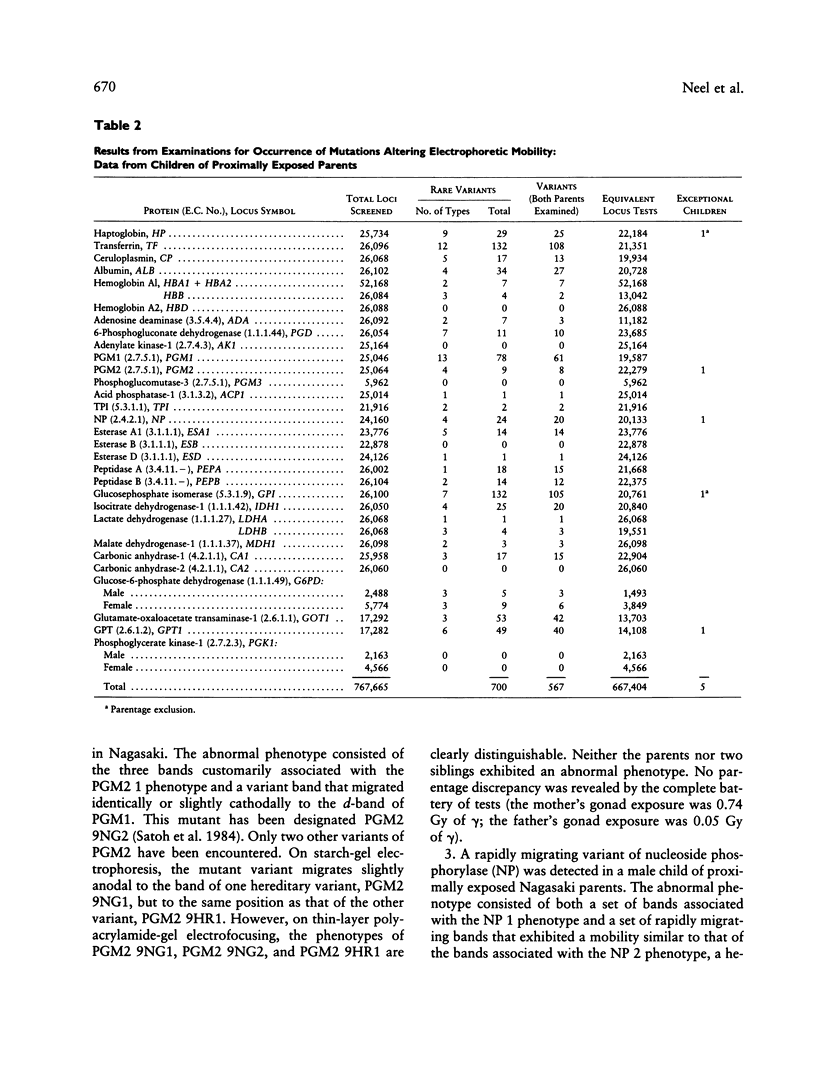
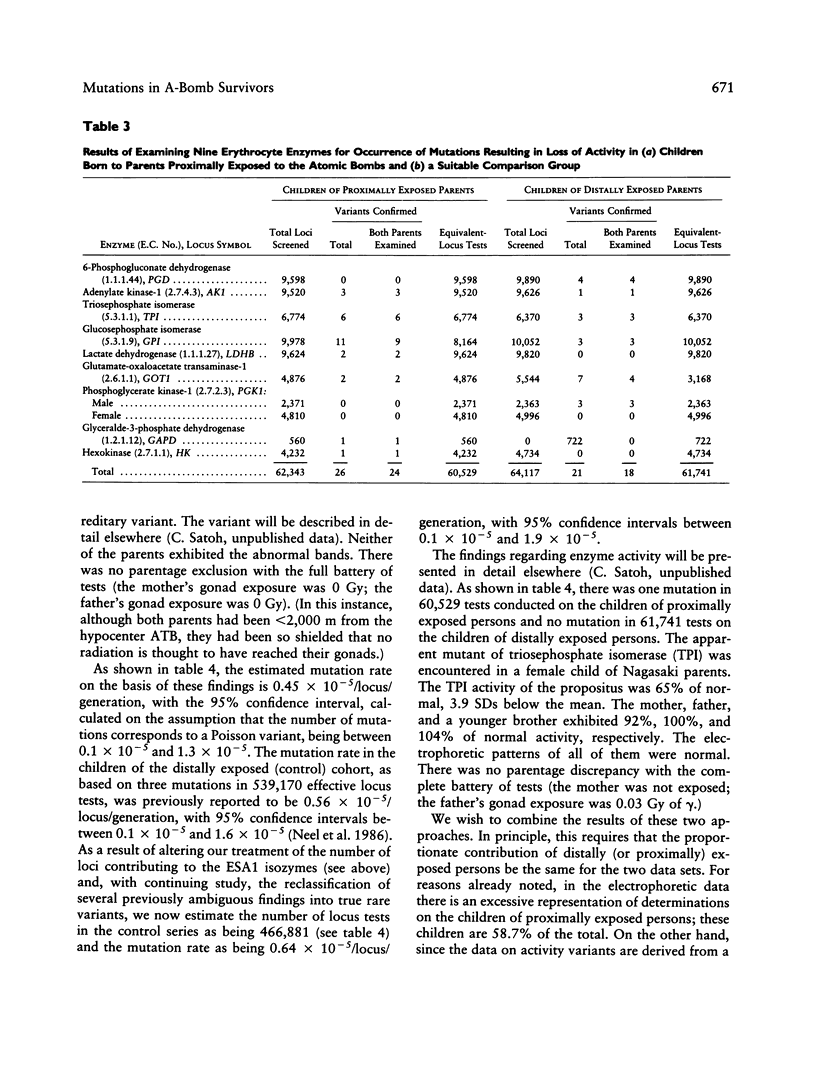
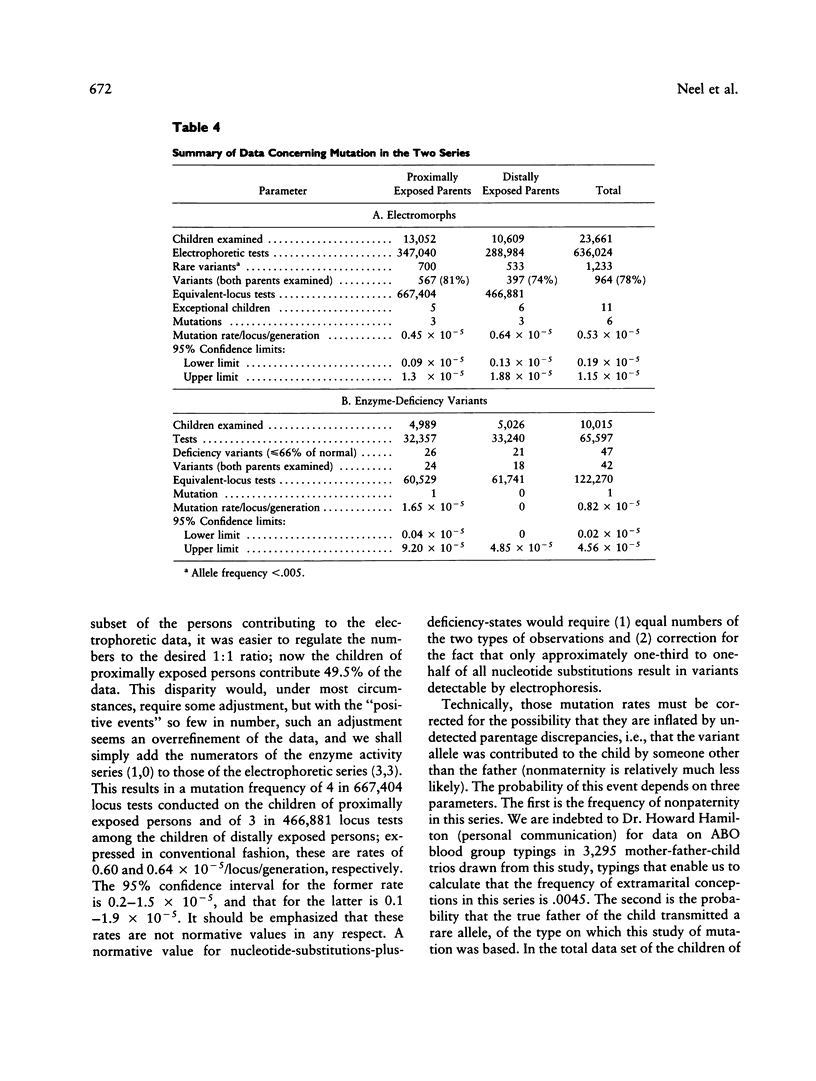
A sample of (1) children whose parents had been proximally exposed (i.e., less than 2,000 m from the hypocenter) at the time of the atomic bombings of Hiroshima and Nagasaki and (2) a suitable comparison group have been examined for the occurrence of mutations altering the electrophoretic mobility or activity of a series of 30 proteins. The examination of the equivalent of 667,404 locus products in the children of proximally exposed persons yielded three mutations altering electrophoretic mobility; the corresponding figure for the comparison group was three mutations in 466,881 tests. The examination of a subset of 60,529 locus products for loss of enzyme activity in the children of proximally exposed persons yielded one mutation; no mutations were encountered in 61,741 determinations on the children of the comparison group. When these two series are compared, the mutation rate observed in the children of proximally exposed persons is thus 0.60 x 10(-5)/locus/generation, with 95% confidence intervals between 0.2 and 1.5 x 10(-5), and that in the comparison children is 0.64 x 10(-5)/locus/generation, with 95% intervals between 0.1 and 1.9 x 10(-5). The average conjoint gonad doses for the proximally exposed parents are estimated to be 0.437 Gy of gamma radiation and 0.002 Gy of neutron radiation. If a relative biological effectiveness of 20 is assigned to the neutron radiation, the combined total gonad dose for the parents becomes 0.477 Sv. (Organ absorbed doses are expressed in gray [1 Gy = 100 rad]; where dose is a mixture of gamma and neutron radiation, it is necessary because of the differing relative biological effectiveness of gamma and neutron radiation to express the combined gamma-neutron gonad exposures in sieverts [1 Sv = 100 rem]).
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertini R. J., O'Neill J. P., Nicklas J. A., Heintz N. H., Kelleher P. C. Alterations of the hprt gene in human in vivo-derived 6-thioguanine-resistant T lymphocytes. Nature. 1985 Jul 25;316(6026):369–371. doi: 10.1038/316369a0. [DOI] [PubMed] [Google Scholar]
- Antonarakis S. E., Kazazian H. H., Jr, Orkin S. H. DNA polymorphism and molecular pathology of the human globin gene clusters. Hum Genet. 1985;69(1):1–14. doi: 10.1007/BF00295521. [DOI] [PubMed] [Google Scholar]
- Ashman C. R., Davidson R. L. Sequence analysis of spontaneous mutations in a shuttle vector gene integrated into mammalian chromosomal DNA. Proc Natl Acad Sci U S A. 1987 May;84(10):3354–3358. doi: 10.1073/pnas.84.10.3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auxier J. A. A physical dose estimates for A-bomb survivors. Studies at Oak Ridge, U.S.A. J Radiat Res. 1975 Sep;16 (Suppl):1–11. doi: 10.1269/jrr.16.supplement_1. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Shaw M., Schull W. J. Exclusion of paternity: the current state of the art. Am J Hum Genet. 1974 Jul;26(4):477–488. [PMC free article] [PubMed] [Google Scholar]
- Delehanty J., White R. L., Mendelsohn M. L. International Commission for Protection Against Environmental Mutagens and Carcinogens. ICPEMC Meeting Report No. 2. Approaches to determining mutation rates in human DNA. Mutat Res. 1986 May;167(3):215–232. doi: 10.1016/0165-1110(86)90031-x. [DOI] [PubMed] [Google Scholar]
- Ferrell R. E., Ueda N., Satoh C., Tanis R. J., Neel J. V., Hamilton H. B., Inamizu T., Baba K. The frequency in Japanese of genetic variants of 22 proteins. I. Albumin, ceruloplasmin, haptoglobin, and transferrin. Ann Hum Genet. 1977 May;40(4):407–418. [PubMed] [Google Scholar]
- Garriott M. L., Grahn D. Neutron and gamma-ray effects measured by the micronucleus test. Mutat Res. 1982 Sep;105(3):157–162. doi: 10.1016/0165-7992(82)90005-7. [DOI] [PubMed] [Google Scholar]
- Gibbs R. A., Caskey C. T. Identification and localization of mutations at the Lesch-Nyhan locus by ribonuclease A cleavage. Science. 1987 Apr 17;236(4799):303–305. doi: 10.1126/science.3563511. [DOI] [PubMed] [Google Scholar]
- Gilbert E. S. Some effects of random dose measurement errors on analyses of atomic bomb survivor data. Radiat Res. 1984 Jun;98(3):591–605. [PubMed] [Google Scholar]
- Grahn D., Carnes B. A., Farrington B. H., Lee C. H. Genetic injury in hybrid male mice exposed to low doses of 60Co gamma-rays or fission neutrons. I. Response to single doses. Mutat Res. 1984 Nov;129(2):215–229. doi: 10.1016/0027-5107(84)90154-4. [DOI] [PubMed] [Google Scholar]
- Grahn D., Lee C. H., Farrington B. F. Interpretation of cytogenetic damage induced in the germ line of male mice exposed for over 1 year to 239Pu alpha particles, fission neutrons, or 60Co gamma rays. Radiat Res. 1983 Sep;95(3):566–583. [PubMed] [Google Scholar]
- Grosovsky A. J., Drobetsky E. A., deJong P. J., Glickman B. W. Southern analysis of genomic alterations in gamma-ray-induced aprt- hamster cell mutants. Genetics. 1986 Jun;113(2):405–415. doi: 10.1093/genetics/113.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosovsky A. J., de Boer J. G., de Jong P. J., Drobetsky E. A., Glickman B. W. Base substitutions, frameshifts, and small deletions constitute ionizing radiation-induced point mutations in mammalian cells. Proc Natl Acad Sci U S A. 1988 Jan;85(1):185–188. doi: 10.1073/pnas.85.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume T., Maruyama T. Physical dose estimates for A-bomb survivors. Studies at Chiba, Japan. J Radiat Res. 1975 Sep;16 (Suppl):12–23. doi: 10.1269/jrr.16.supplement_12. [DOI] [PubMed] [Google Scholar]
- Ito H., Yasuda N., Matsumoto H. The probability of parentage exclusion based on restriction fragment length polymorphisms. Jinrui Idengaku Zasshi. 1985 Dec;30(4):261–269. doi: 10.1007/BF01907963. [DOI] [PubMed] [Google Scholar]
- Lewis S. E., Johnson F. M. The nature of spontaneous and induced electrophoretically detected mutations in the mouse. Prog Clin Biol Res. 1986;209B:359–365. [PubMed] [Google Scholar]
- Liber H. L., Call K. M., Little J. B. Molecular and biochemical analyses of spontaneous and X-ray-induced mutants in human lymphoblastoid cells. Mutat Res. 1987 May;178(1):143–153. doi: 10.1016/0027-5107(87)90096-0. [DOI] [PubMed] [Google Scholar]
- Liber H. L., Leong P. M., Terry V. H., Little J. B. X-rays mutate human lymphoblast cells at genetic loci that should respond only to point mutagens. Mutat Res. 1986 Oct;163(1):91–97. doi: 10.1016/0027-5107(86)90062-x. [DOI] [PubMed] [Google Scholar]
- Malling H. V., De Serres F. J. Genetic alterations at the molecular level in x-ray induced ad-3B mutants of Neurospora crassa. Radiat Res. 1973 Jan;53(1):77–87. [PubMed] [Google Scholar]
- Malling H. V., Valcovic L. R. A biochemical specific locus mutation system in mice. Arch Toxicol. 1977 Sep 21;38(1-2):45–51. doi: 10.1007/BF00293662. [DOI] [PubMed] [Google Scholar]
- Mohrenweiser H. W. Frequency of enzyme deficiency variants in erythrocytes of newborn infants. Proc Natl Acad Sci U S A. 1981 Aug;78(8):5046–5050. doi: 10.1073/pnas.78.8.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Colletti-Feener C., Kunkel L. M. Localization and cloning of Xp21 deletion breakpoints involved in muscular dystrophy. Hum Genet. 1987 Mar;75(3):221–227. doi: 10.1007/BF00281063. [DOI] [PubMed] [Google Scholar]
- Neel J. V., Kato H., Schull W. J. Mortality in the children of atomic bomb survivors and controls. Genetics. 1974 Feb;76(2):311–336. doi: 10.1093/genetics/76.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Satoh C., Goriki K., Fujita M., Takahashi N., Asakawa J., Hazama R. The rate with which spontaneous mutation alters the electrophoretic mobility of polypeptides. Proc Natl Acad Sci U S A. 1986 Jan;83(2):389–393. doi: 10.1073/pnas.83.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Satoh C., Hamilton H. B., Otake M., Goriki K., Kageoka T., Fujita M., Neriishi S., Asakawa J. Search for mutations affecting protein structure in children of atomic bomb survivors: preliminary report. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4221–4225. doi: 10.1073/pnas.77.7.4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neel J. V., Ueda N., Satoh C., Ferrell R. E., Tanis R. J., Hamilton H. B. The frequency in Japanese of genetic variants of 22 proteins. V. Summary and comparison with data on Caucasians from the British Isles. Ann Hum Genet. 1978 May;41(4):429–441. doi: 10.1111/j.1469-1809.1978.tb00913.x. [DOI] [PubMed] [Google Scholar]
- RUSSELL W. L. X-ray-induced mutations in mice. Cold Spring Harb Symp Quant Biol. 1951;16:327–336. doi: 10.1101/sqb.1951.016.01.024. [DOI] [PubMed] [Google Scholar]
- Rinchik E. M., Russell L. B., Copeland N. G., Jenkins N. A. Molecular genetic analysis of the dilute-short ear (d-se) region of the mouse. Genetics. 1986 Feb;112(2):321–342. doi: 10.1093/genetics/112.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. B. Information from specific-locus mutants on the nature of induced and spontaneous mutations in the mouse. Prog Clin Biol Res. 1986;209B:437–447. [PubMed] [Google Scholar]
- Russell L. B., Russell W. L., Popp R. A., Vaughan C., Jacobson K. B. Radiation-induced mutations at mouse hemoglobin loci. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2843–2846. doi: 10.1073/pnas.73.8.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh C., Awa A. A., Neel J. V., Schull W. J., Kato H., Hamilton H. B., Otake M., Goriki K. Genetic effects of atomic bombs. Prog Clin Biol Res. 1982;103(Pt A):267–276. [PubMed] [Google Scholar]
- Satoh C., Ferrell R. E., Tanis R. J., Ueda N., Kishimoto S., Neel J. V., Hamilton H. B., Baba K. The frequency in Japanese of genetic variants of 22 proteins. III. Phosphoglucomutase-1, phosphoglucomutase-2, 6-phosphogluconate dehydrogenase, adenylate kinase, and adenosine deaminase. Ann Hum Genet. 1977 Oct;41(2):169–183. doi: 10.1111/j.1469-1809.1977.tb01912.x. [DOI] [PubMed] [Google Scholar]
- Satoh C., Neel J. V., Yamashita A., Goriki K., Fujita M., Hamilton H. B. The frequency among Japanese of heterozygotes for deficiency variants of 11 enzymes. Am J Hum Genet. 1983 Jul;35(4):656–674. [PMC free article] [PubMed] [Google Scholar]
- Satoh C., Takahashi N., Asakawa J., Masunari N., Fujita M., Goriki K., Hazama R., Iwamoto K. Electrophoretic variants of blood proteins in Japanese. I. Phosphoglucomutase-2 (PGM2). Jinrui Idengaku Zasshi. 1984 Jun;29(2):89–104. doi: 10.1007/BF01873529. [DOI] [PubMed] [Google Scholar]
- Schull W. J., Otake M., Neel J. V. Genetic effects of the atomic bombs: a reappraisal. Science. 1981 Sep 11;213(4513):1220–1227. doi: 10.1126/science.7268429. [DOI] [PubMed] [Google Scholar]
- Skolnick M. M., Neel J. V. An algorithm for comparing two-dimensional electrophoretic gels, with particular reference to the study of mutation. Adv Hum Genet. 1986;15:55–160. doi: 10.1007/978-1-4615-8356-1_2. [DOI] [PubMed] [Google Scholar]
- Stout J. T., Caskey C. T. HPRT: gene structure, expression, and mutation. Annu Rev Genet. 1985;19:127–148. doi: 10.1146/annurev.ge.19.120185.001015. [DOI] [PubMed] [Google Scholar]
- Tanis R. J., Ueda N., Satoh C., Ferrell R. E., Kishi-Moto S., Neel J. V., Hamilton H. B., Ohno N. The frequency in Japanese of genetic variants of 22 proteins. IV. Acid phosphatase, NADP-isocitrate dehydrogenase, peptidase A, peptidase B and phosphohexose isomerase. Ann Hum Genet. 1978 May;41(4):419–428. doi: 10.1111/j.1469-1809.1978.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Turner D. R., Morley A. A., Haliandros M., Kutlaca R., Sanderson B. J. In vivo somatic mutations in human lymphocytes frequently result from major gene alterations. Nature. 1985 May 23;315(6017):343–345. doi: 10.1038/315343a0. [DOI] [PubMed] [Google Scholar]
- Ueda N., Satoh C., Tanis R. J., Ferrell R. E., Kishimoto S., Neel J. V., Hamilton H. B., Baba K. The frequency in Japanese of genetic variants of 22 proteins II. Carbonic anhydrase I and II, lactate dehydrogenase, malate dehydrogenase, nucleoside phosphorylase, triose phosphate isomerase, haemoglobin A and haemoglobin A2. Ann Hum Genet. 1977 Jul;41(1):43–52. doi: 10.1111/j.1469-1809.1977.tb01960.x. [DOI] [PubMed] [Google Scholar]
- Wilson J. M., Stout J. T., Palella T. D., Davidson B. L., Kelley W. N., Caskey C. T. A molecular survey of hypoxanthine-guanine phosphoribosyltransferase deficiency in man. J Clin Invest. 1986 Jan;77(1):188–195. doi: 10.1172/JCI112275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yandell D. W., Dryja T. P., Little J. B. Somatic mutations at a heterozygous autosomal locus in human cells occur more frequently by allele loss than by intragenic structural alterations. Somat Cell Mol Genet. 1986 May;12(3):255–263. doi: 10.1007/BF01570784. [DOI] [PubMed] [Google Scholar]
- Youssoufian H., Antonarakis S. E., Aronis S., Tsiftis G., Phillips D. G., Kazazian H. H., Jr Characterization of five partial deletions of the factor VIII gene. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3772–3776. doi: 10.1073/pnas.84.11.3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ommen G. J., Verkerk J. M., Hofker M. H., Monaco A. P., Kunkel L. M., Ray P., Worton R., Wieringa B., Bakker E., Pearson P. L. A physical map of 4 million bp around the Duchenne muscular dystrophy gene on the human X-chromosome. Cell. 1986 Nov 21;47(4):499–504. doi: 10.1016/0092-8674(86)90614-8. [DOI] [PubMed] [Google Scholar]


