Abstract
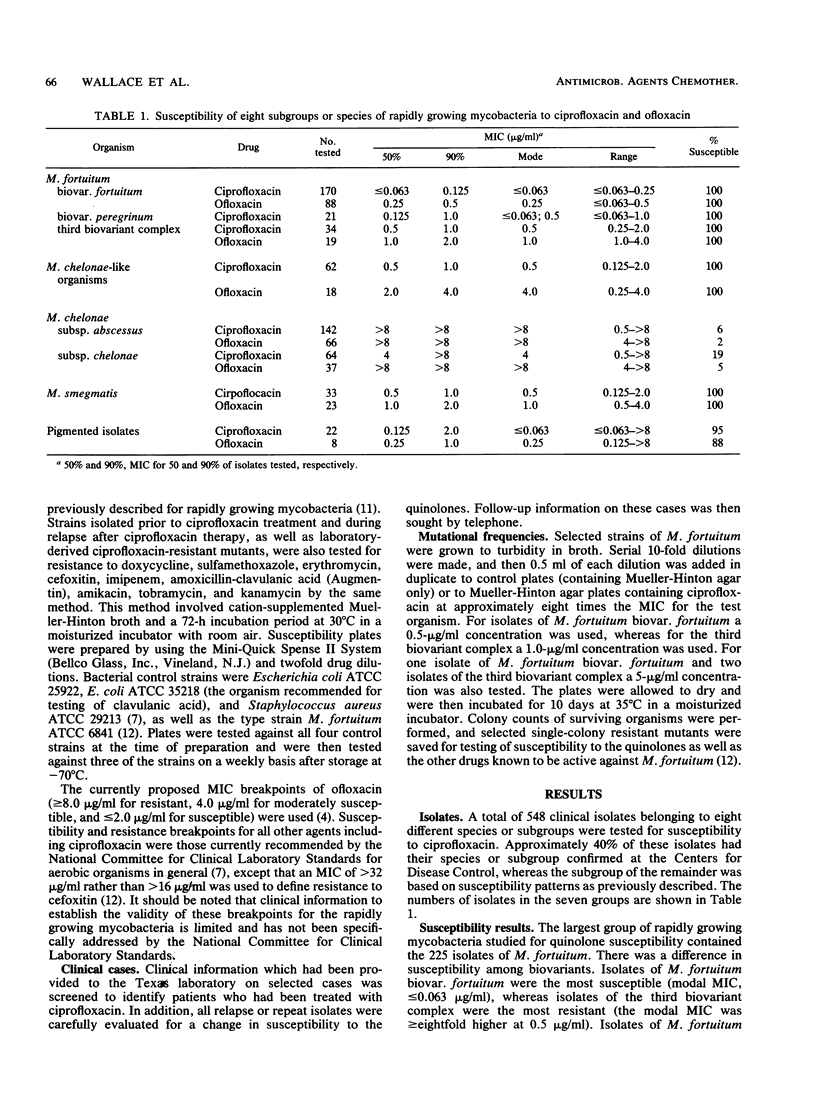
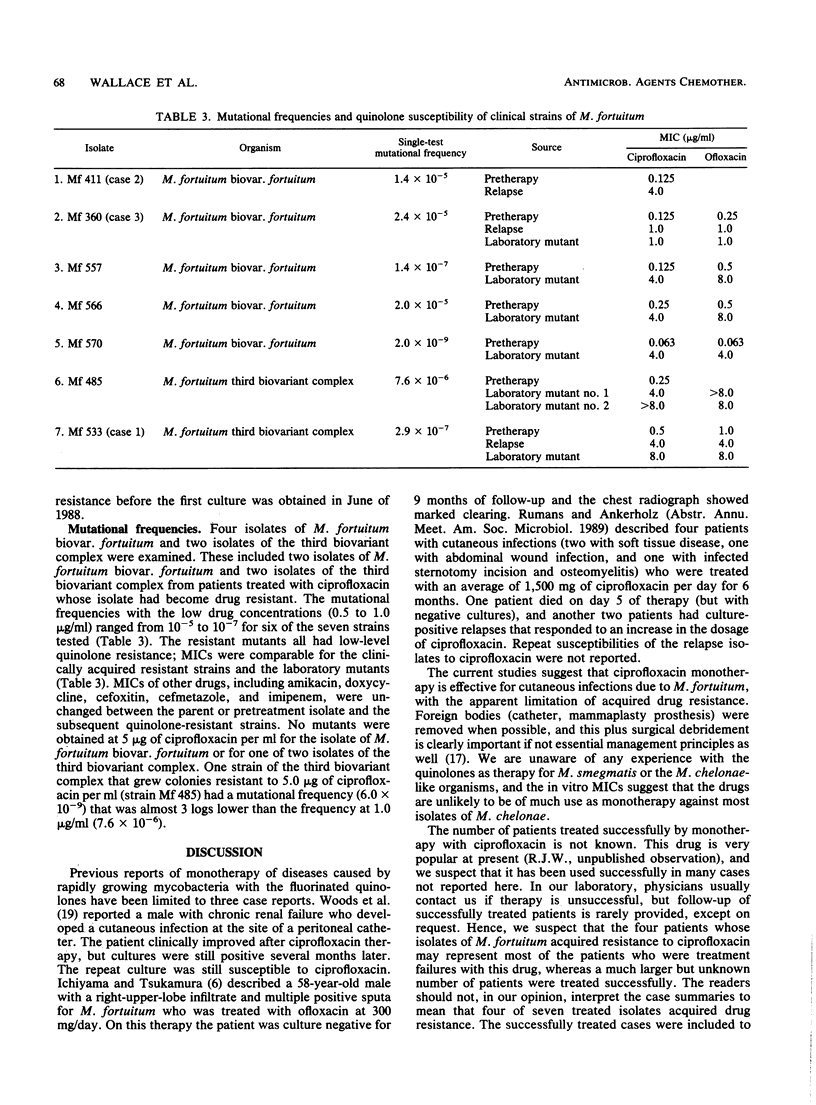
The susceptibility to ciprofloxacin of 548 clinical isolates of rapidly growing mycobacteria belonging to eight subgroups or species was determined. The 170 isolates of Mycobacterium fortuitum biovar.fortuitum were most susceptible; the MIC for 90% of the organisms was 0.125 micrograms/ml. The other biovariants of M. fortuitum, M. smegmatis, and the M. chelonae-like organisms were less susceptible; the modal MIC was 0.5 micrograms/ml, and the MIC for 90% of organisms was 1.0 micrograms/ml. The two subspecies of M. chelonae were generally resistant, with only 8% of 206 isolates falling in the moderately susceptible category (MIC, 2 micrograms/ml) and only 2% falling in the susceptible category (MIC, less than or equal to 1 micrograms/ml). MICs of ofloxacin averaged 1 to 2 dilutions higher than those of ciprofloxacin for all subgroups tested. Three patients with M. fortuitum cutaneous disease relapsed after an initial response to therapy with ciprofloxacin, and their isolate was shown to have acquired drug resistance. Mutational frequencies for M. fortuitum with ciprofloxacin were relatively high (10(-5) to 10(-7), and MICs for single-step mutants were similar to those for the clinically resistant strains. Thus, despite the excellent activity of ciprofloxacin against rapidly growing mycobacterial groups other than M. chelonae, single-drug therapy should be used with caution because of the risk of development of mutational resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Casal M. J., Rodriguez F. C. Simple, new test for rapid differentiation of the Mycobacterium fortuitum complex. J Clin Microbiol. 1981 May;13(5):989–990. doi: 10.1128/jcm.13.5.989-990.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins C. H., Yates M. D., Uttley A. H. Differentiation of Mycobacterium chelonei from M. fortuitum by ciprofloxacin susceptibility. J Hyg (Lond) 1985 Dec;95(3):619–621. doi: 10.1017/s002217240006071x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalovisio J. R., Pankey G. A., Wallace R. J., Jones D. B. Clinical usefulness of amikacin and doxycycline in the treatment of infection due to Mycobacterium fortuitum and Mycobacterium chelonei. Rev Infect Dis. 1981 Sep-Oct;3(5):1068–1074. doi: 10.1093/clinids/3.5.1068. [DOI] [PubMed] [Google Scholar]
- Fuchs P. C., Barry A. L., Jones R. N., Thornsberry C. Proposed disk diffusion susceptibility criteria for ofloxacin. J Clin Microbiol. 1985 Aug;22(2):310–311. doi: 10.1128/jcm.22.2.310-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay J. D., DeYoung D. R., Roberts G. D. In vitro activities of norfloxacin and ciprofloxacin against Mycobacterium tuberculosis, M. avium complex, M. chelonei, M. fortuitum, and M. kansasii. Antimicrob Agents Chemother. 1984 Jul;26(1):94–96. doi: 10.1128/aac.26.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama S., Tsukamura M. Ofloxacin and the treatment of pulmonary disease due to Mycobacterium fortuitum. Chest. 1987 Dec;92(6):1110–1112. doi: 10.1378/chest.92.6.1110. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V., Werner V. Selection of multiple antibiotic resistance by quinolones, beta-lactams, and aminoglycosides with special reference to cross-resistance between unrelated drug classes. Antimicrob Agents Chemother. 1984 Dec;26(6):797–801. doi: 10.1128/aac.26.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silcox V. A., Good R. C., Floyd M. M. Identification of clinically significant Mycobacterium fortuitum complex isolates. J Clin Microbiol. 1981 Dec;14(6):686–691. doi: 10.1128/jcm.14.6.686-691.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele L. C., Wallace R. J., Jr Ability of ciprofloxacin but not pipemidic acid to differentiate all three biovariants of Mycobacterium fortuitum from Mycobacterium chelonae. J Clin Microbiol. 1987 Feb;25(2):456–457. doi: 10.1128/jcm.25.2.456-457.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Thornsberry C., Silcox V. A. Rapidly growing mycobacteria: testing of susceptibility to 34 antimicrobial agents by broth microdilution. Antimicrob Agents Chemother. 1982 Aug;22(2):186–192. doi: 10.1128/aac.22.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson J. M., Wallace R. J., Jr, Silcox V. A., Thornsberry C. Antimicrobial susceptibility of five subgroups of Mycobacterium fortuitum and Mycobacterium chelonae. Antimicrob Agents Chemother. 1985 Dec;28(6):807–811. doi: 10.1128/aac.28.6.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamura M. In vitro antimycobacterial activity of a new antibacterial substance DL-8280--differentiation between some species of mycobacteria and related organisms by the DL-8280 susceptibility test. Microbiol Immunol. 1983;27(12):1129–1132. doi: 10.1111/j.1348-0421.1983.tb02933.x. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Jones D. B., Wiss K. Sulfonamide activity against Mycobacterium fortuitum and Mycobacterium chelonei. Rev Infect Dis. 1981 Sep-Oct;3(5):898–904. doi: 10.1093/clinids/3.5.898. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Nash D. R., Tsukamura M., Blacklock Z. M., Silcox V. A. Human disease due to Mycobacterium smegmatis. J Infect Dis. 1988 Jul;158(1):52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Bullen M. G. Treatment of nonpulmonary infections due to Mycobacterium fortuitum and Mycobacterium chelonei on the basis of in vitro susceptibilities. J Infect Dis. 1985 Sep;152(3):500–514. doi: 10.1093/infdis/152.3.500. [DOI] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Swenson J. M., Silcox V. A., Good R. C., Tschen J. A., Stone M. S. Spectrum of disease due to rapidly growing mycobacteria. Rev Infect Dis. 1983 Jul-Aug;5(4):657–679. doi: 10.1093/clinids/5.4.657. [DOI] [PubMed] [Google Scholar]
- Woods G. L., Hall G. S., Schreiber M. J. Mycobacterium fortuitum peritonitis associated with continuous ambulatory peritoneal dialysis. J Clin Microbiol. 1986 Apr;23(4):786–788. doi: 10.1128/jcm.23.4.786-788.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yew W. W., Kwan S. Y., Ma W. K., Aung-khin M., Mok C. K. Combination of ofloxacin and amikacin in the treatment of sternotomy wound infection. Chest. 1989 May;95(5):1051–1055. doi: 10.1378/chest.95.5.1051. [DOI] [PubMed] [Google Scholar]
- Young L. S., Berlin O. G., Inderlied C. B. Activity of ciprofloxacin and other fluorinated quinolones against mycobacteria. Am J Med. 1987 Apr 27;82(4A):23–26. [PubMed] [Google Scholar]


