Abstract
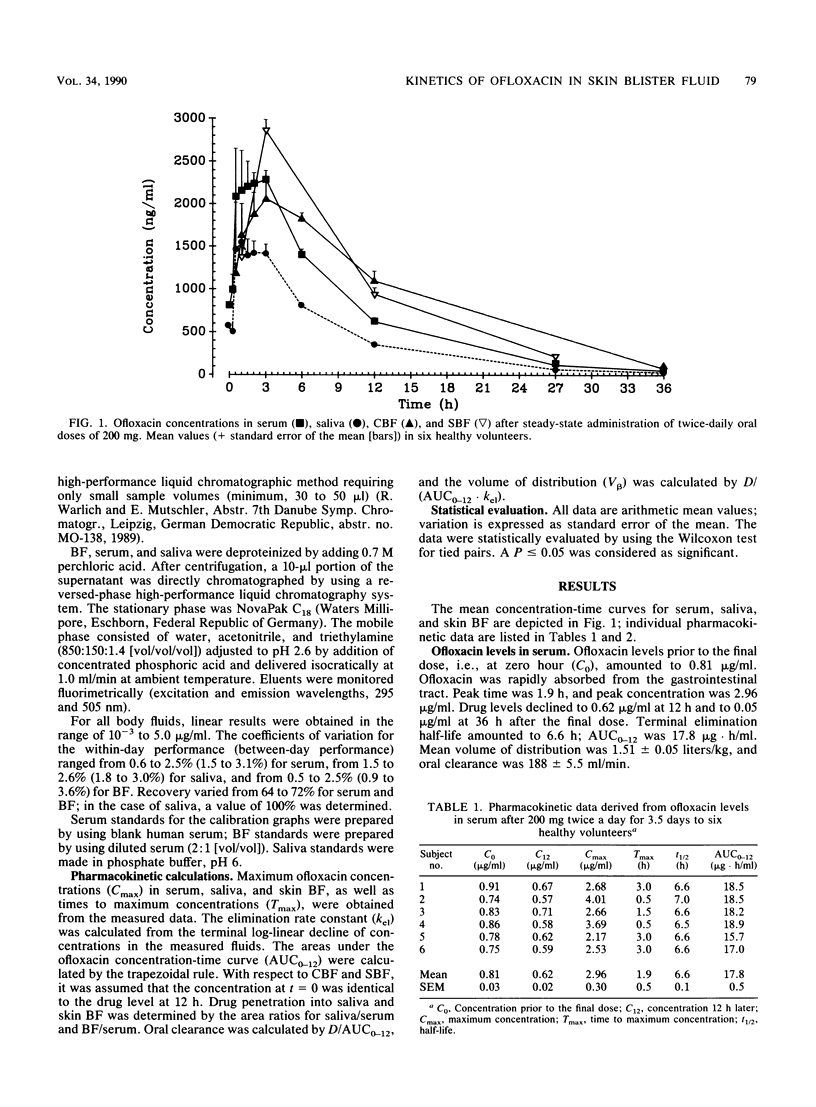
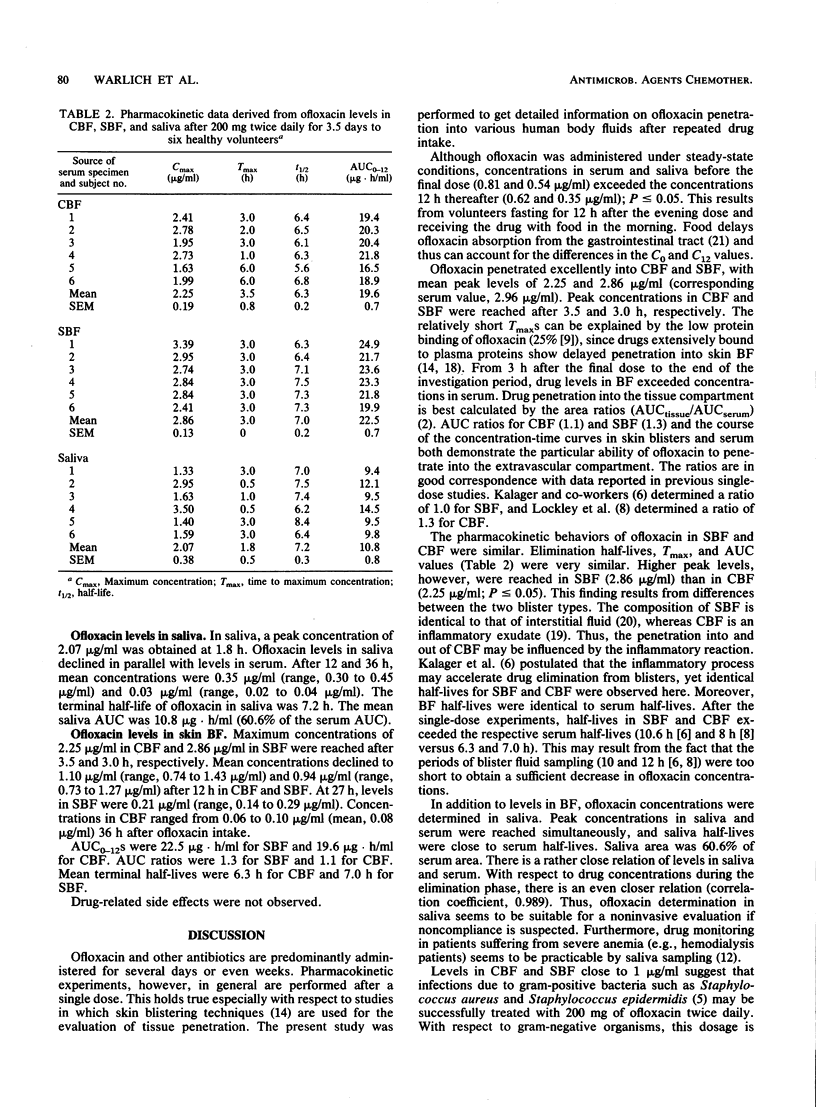
The pharmacokinetics of ofloxacin were determined in six healthy volunteers after oral administration of 200 mg twice daily for 3.5 days. To study the pharmacokinetic behavior at the target site in bacterial infection of the skin, drug concentrations were determined in suction blister fluid (SBF) and cantharides blister fluid (CBF), as well as in serum and saliva. Ofloxacin was measured by a high-performance liquid chromatographic assay. Ofloxacin concentrations in saliva amounted to 61 +/- 3% of levels in serum. After the final dose, ofloxacin concentrations in blister fluid and serum declined in parallel. Terminal half-lives of ofloxacin in blister fluids (SBF, 7.0 h; CBF, 6.3 h) were in accordance with serum half-life (6.6 h). Favorable penetration into the skin is suggested by high area under the concentration-time curve ratios for blister fluid and serum (CBF, 1.1; SBF, 1.3). During repeated ofloxacin intake, drug levels in SBF and CBF at 12 h amounted to 0.94 and 1.10 micrograms/ml. Thus, ofloxacin levels in the skin are well above the MIC for 90% of strains tested for, e.g., Staphylococcus aureus, Staphylococcus epidermidis, Neisseria gonorrhoeae, and various members of the family Enterobacteriaceae. This should also hold true with respect to other tissues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLISON J. H., BETTLEY F. R. Investigations into cantharidin blisters raised on apparently normal skin in normal and abnormal subjects. Br J Dermatol. 1958 Oct;70(10):331–339. doi: 10.1111/j.1365-2133.1958.tb13792.x. [DOI] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetics of tissue penetration of antibiotics. Rev Infect Dis. 1981 Jan-Feb;3(1):45–66. doi: 10.1093/clinids/3.1.45. [DOI] [PubMed] [Google Scholar]
- Chin N. X., Brittain D. C., Neu H. C. In vitro activity of Ro 23-6240, a new fluorinated 4-quinolone. Antimicrob Agents Chemother. 1986 Apr;29(4):675–680. doi: 10.1128/aac.29.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W., Stieglitz M., Baars B., Opferkuch W. Comparative evaluation of recently developed quinolone compounds--with a note on the frequency of resistant mutants. Chemotherapy. 1985;31(1):19–28. doi: 10.1159/000238309. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Hosaka M., Oomori Y., Niwata Y., Suzue S., Irikura T. In vitro and in vivo antibacterial activity of AM-833, a new quinolone derivative. Antimicrob Agents Chemother. 1986 Jun;29(6):1059–1066. doi: 10.1128/aac.29.6.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalager T., Digranes A., Bergan T., Rolstad T. Ofloxacin: serum and skin blister fluid pharmacokinetics in the fasting and non-fasting state. J Antimicrob Chemother. 1986 Jun;17(6):795–800. doi: 10.1093/jac/17.6.795. [DOI] [PubMed] [Google Scholar]
- Kiistala U. Suction blister device for separation of viable epidermis from dermis. J Invest Dermatol. 1968 Feb;50(2):129–137. doi: 10.1038/jid.1968.15. [DOI] [PubMed] [Google Scholar]
- Lockley M. R., Wise R., Dent J. The pharmacokinetics and tissue penetration of ofloxacin. J Antimicrob Chemother. 1984 Dec;14(6):647–652. doi: 10.1093/jac/14.6.647. [DOI] [PubMed] [Google Scholar]
- Lode H., Höffken G., Olschewski P., Sievers B., Kirch A., Borner K., Koeppe P. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob Agents Chemother. 1987 Sep;31(9):1338–1342. doi: 10.1128/aac.31.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manek N., Andrews J. M., Wise R. In vitro activity of Ro 23-6240, a new difluoroquinolone derivative, compared with that of other antimicrobial agents. Antimicrob Agents Chemother. 1986 Aug;30(2):330–332. doi: 10.1128/aac.30.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk J. P., Campoli-Richards D. M. Ofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1987 Apr;33(4):346–391. doi: 10.2165/00003495-198733040-00003. [DOI] [PubMed] [Google Scholar]
- Neuman M. Clinical pharmacokinetics of the newer antibacterial 4-quinolones. Clin Pharmacokinet. 1988 Feb;14(2):96–121. doi: 10.2165/00003088-198814020-00003. [DOI] [PubMed] [Google Scholar]
- Schäfer-Korting M., Korting H. C., Hiemstra S., Mutschler E. Does cantharides blister fluid provide access to the peripheral compartment? Eur J Clin Pharmacol. 1982 Oct;23(4):327–330. doi: 10.1007/BF00613614. [DOI] [PubMed] [Google Scholar]
- Schäfer-Korting M., Korting H. C., Maass L., Klesel N., Grigoleit H. G., Mutschler E. Cefodizime penetration into skin suction blister fluid following a single intravenous dose. Eur J Clin Pharmacol. 1986;30(3):295–298. doi: 10.1007/BF00541531. [DOI] [PubMed] [Google Scholar]
- Schäfer-Korting M., Korting H. C., Mutschler E. Human plasma and skin blister fluid levels of griseofulvin following a single oral dose. Eur J Clin Pharmacol. 1985;29(1):109–113. doi: 10.1007/BF00547378. [DOI] [PubMed] [Google Scholar]
- Shyu W. C., Quintiliani R., Nightingale C. H., Dudley M. N. Effect of protein binding on drug penetration into blister fluid. Antimicrob Agents Chemother. 1988 Jan;32(1):128–130. doi: 10.1128/aac.32.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verho M., Malerczyk V., Dagrosa E., Korn A. The effect of food on the pharmacokinetics of ofloxacin. Curr Med Res Opin. 1986;10(3):166–171. doi: 10.1185/03007998609110436. [DOI] [PubMed] [Google Scholar]
- Vermeer B. J., Reman F. C., van Gent C. M. The determination of lipids and proteins in suction blister fluid. J Invest Dermatol. 1979 Oct;73(4):303–305. doi: 10.1111/1523-1747.ep12531833. [DOI] [PubMed] [Google Scholar]
- Warlich R., Mutschler E. Thin-layer chromatographic separation and in situ fluorimetric determination of ofloxacin in plasma and pleural fluid. J Chromatogr. 1989 May 30;490(2):395–403. doi: 10.1016/s0378-4347(00)82797-8. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M., Danks G. In-vitro activity of enoxacin (CL-919), a new quinoline derivative, compared with that of other antimicrobial agents. J Antimicrob Chemother. 1984 Mar;13(3):237–244. doi: 10.1093/jac/13.3.237. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. The fluoroquinolones: structures, mechanisms of action and resistance, and spectra of activity in vitro. Antimicrob Agents Chemother. 1985 Oct;28(4):581–586. doi: 10.1128/aac.28.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]


