Abstract
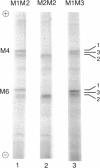
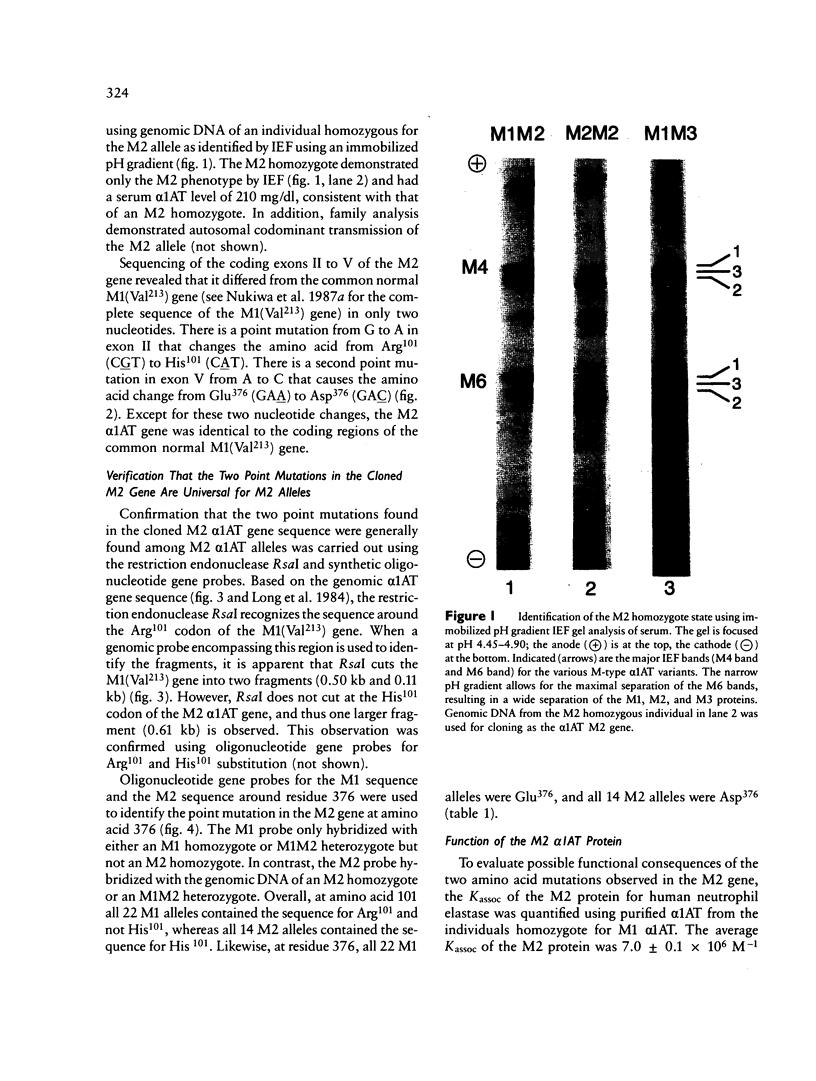
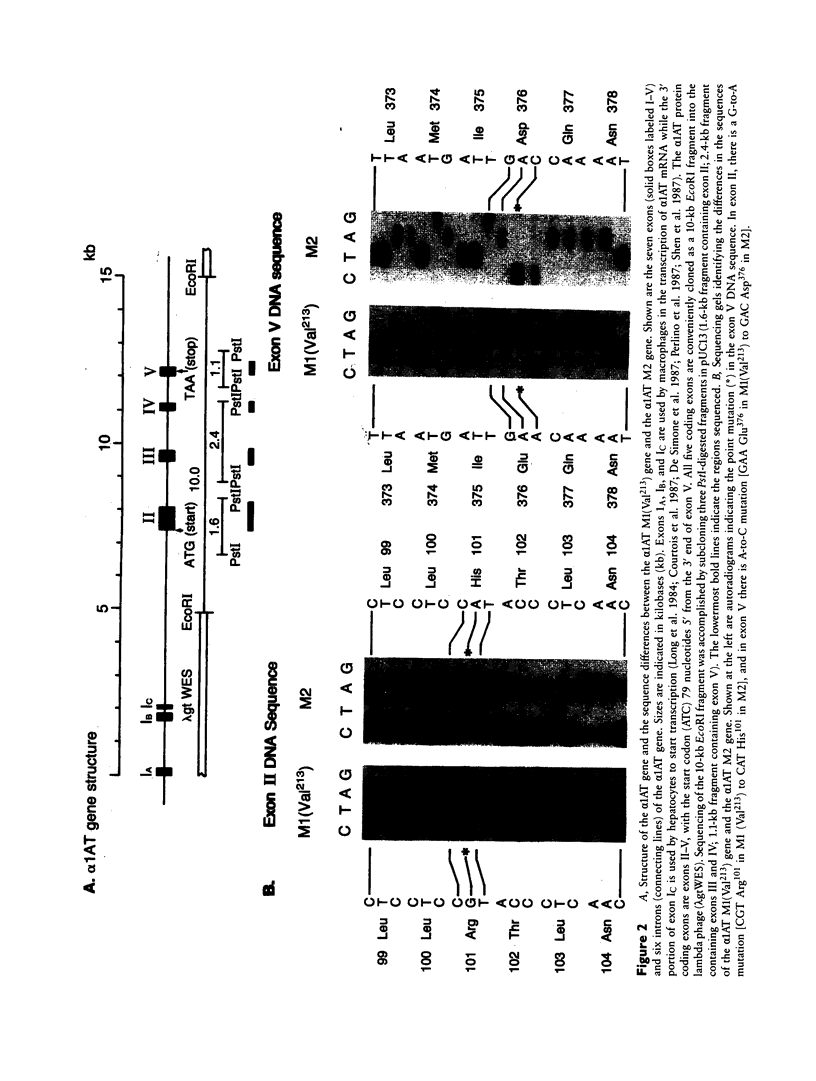
The normal M2 variant of alpha 1-antitrypsin (alpha 1AT) was cloned from a genomic DNA library of an individual homozygous for this allele. Sequencing of all coding exons of the M2 gene revealed it was identical to the common M1(Val213) gene except for two bases (M1(Val213) CGT Arg101, M2 CAT His101; M1(Val213) GAA Glu376 M2 GAC Asp376). Analysis of the sequence of the M1(Val213) and M2 genes around residue 101 revealed the M1 Arg101----M2 His101 caused a loss of the cutting site for the restriction endonuclease RsaI. Using this enzyme, as well as 19-mer oligonucleotides probes centered at residues 101 and 376, evaluation of genomic DNA from 22 M1 alleles and 14 M2 alleles revealed that residue 101 was Arg in all M1 alleles and His in all M2 alleles, while residue 376 was Glu in all M1 alleles and Asp in all M2 alleles. Despite the differences in sequence at two amino acids, the M1(Val213) and M2 proteins function similarly as assessed by quantification of the association rate constant of each for their natural substrate neutrophil elastase. In the context that there are two mutations separating the M1(Val213) and M2 alleles, it is likely that there is another alpha 1AT variant that was an intermediate in the evolution of these genes.
Full text
PDF

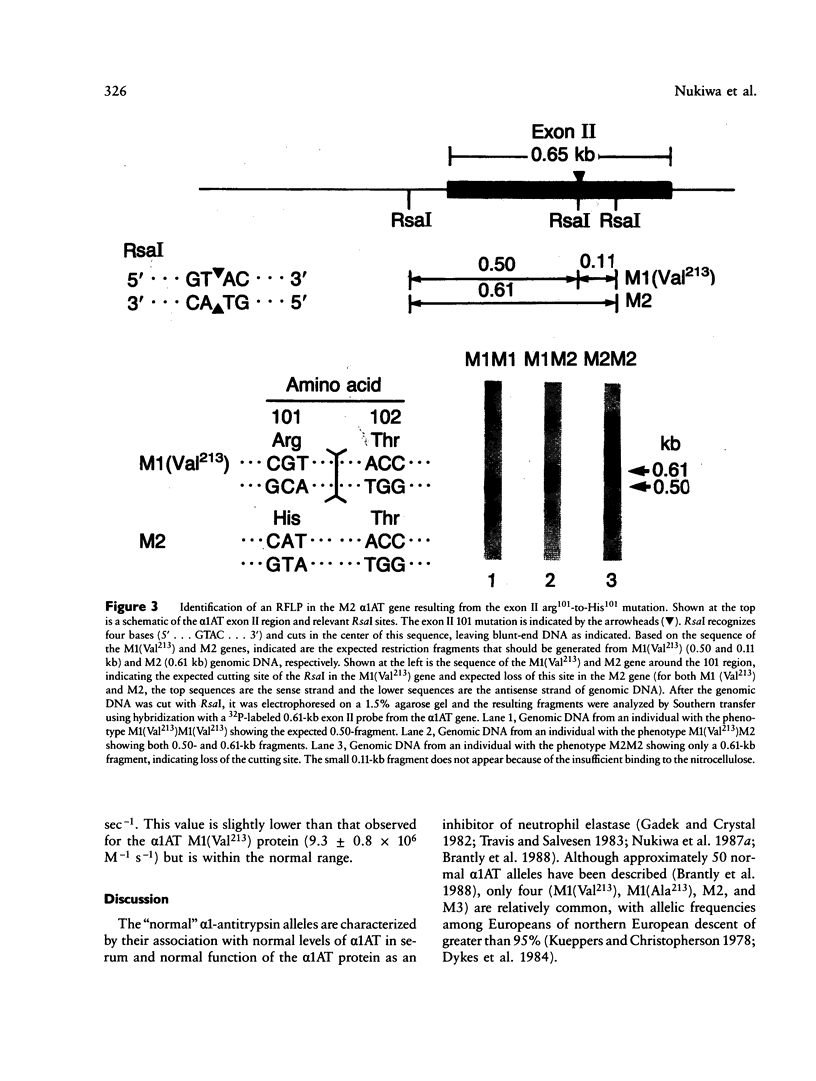
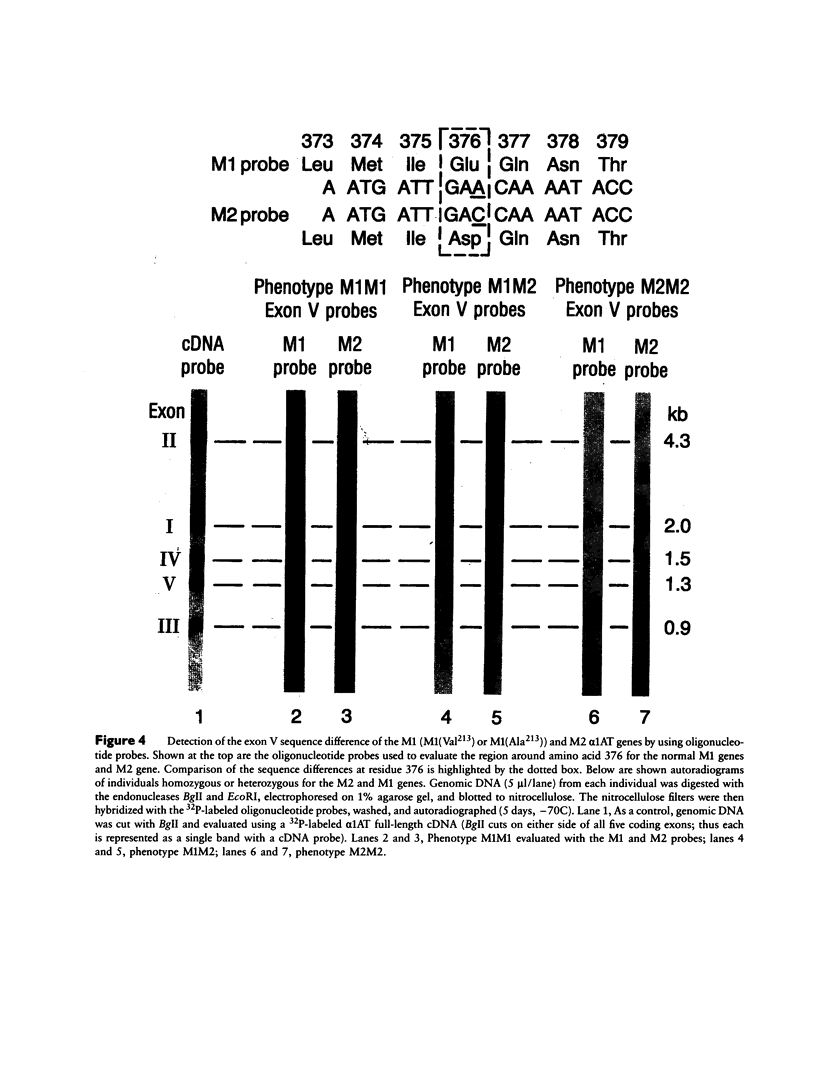



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. C., Harley R. A., Talamo R. C. A new method for determination of alpha-1-antitrypsin phenotypes using isoelectric focusing on polyacrylamide gel slabs. Am J Clin Pathol. 1974 Dec;62(6):732–739. doi: 10.1093/ajcp/62.6.732. [DOI] [PubMed] [Google Scholar]
- Beatty K., Bieth J., Travis J. Kinetics of association of serine proteinases with native and oxidized alpha-1-proteinase inhibitor and alpha-1-antichymotrypsin. J Biol Chem. 1980 May 10;255(9):3931–3934. [PubMed] [Google Scholar]
- Brantly M., Nukiwa T., Crystal R. G. Molecular basis of alpha-1-antitrypsin deficiency. Am J Med. 1988 Jun 24;84(6A):13–31. doi: 10.1016/0002-9343(88)90154-4. [DOI] [PubMed] [Google Scholar]
- Brennan S. O., Carrell R. W. alpha 1-Antitrypsin Christchurch, 363 Glu----Lys: mutation at the P'5 position does not affect inhibitory activity. Biochim Biophys Acta. 1986 Sep 5;873(1):13–19. doi: 10.1016/0167-4838(86)90183-4. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Laurell C. B., Brennan S. O., Owen M. C., Vaughan L., Boswell D. R. Structure and variation of human alpha 1-antitrypsin. Nature. 1982 Jul 22;298(5872):329–334. doi: 10.1038/298329a0. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Jeppsson J. O., Vaughan L., Brennan S. O., Owen M. C., Boswell D. R. Human alpha 1-antitrypsin: carbohydrate attachment and sequence homology. FEBS Lett. 1981 Dec 7;135(2):301–303. doi: 10.1016/0014-5793(81)80805-8. [DOI] [PubMed] [Google Scholar]
- Colau B., Chuchana P., Bollen A. Revised sequence of full-length complementary DNA coding for human alpha 1-antitrypsin. DNA. 1984 Aug;3(4):327–330. doi: 10.1089/dna.1.1984.3.327. [DOI] [PubMed] [Google Scholar]
- Constans J., Viau M., Gouaillard C. Pi M4: an additional Pi M subtype. Hum Genet. 1980;55(1):119–121. doi: 10.1007/BF00329137. [DOI] [PubMed] [Google Scholar]
- Courtney M., Buchwalder A., Tessier L. H., Jaye M., Benavente A., Balland A., Kohli V., Lathe R., Tolstoshev P., Lecocq J. P. High-level production of biologically active human alpha 1-antitrypsin in Escherichia coli. Proc Natl Acad Sci U S A. 1984 Feb;81(3):669–673. doi: 10.1073/pnas.81.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtois G., Morgan J. G., Campbell L. A., Fourel G., Crabtree G. R. Interaction of a liver-specific nuclear factor with the fibrinogen and alpha 1-antitrypsin promoters. Science. 1987 Oct 30;238(4827):688–692. doi: 10.1126/science.3499668. [DOI] [PubMed] [Google Scholar]
- Cox D. W., Johnson A. M., Fagerhol M. K. Report of Nomenclature Meeting for alpha 1-antitrypsin, INSERM, Rouen/Bois-Guillaume-1978. Hum Genet. 1980;53(3):429–433. doi: 10.1007/BF00287070. [DOI] [PubMed] [Google Scholar]
- De Simone V., Ciliberto G., Hardon E., Paonessa G., Palla F., Lundberg L., Cortese R. Cis- and trans-acting elements responsible for the cell-specific expression of the human alpha 1-antitrypsin gene. EMBO J. 1987 Sep;6(9):2759–2766. doi: 10.1002/j.1460-2075.1987.tb02570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykes D. D., Miller S. A., Polesky H. F. Distribution of alpha 1-antitrypsin variants in a US white population. Hum Hered. 1984;34(5):308–310. doi: 10.1159/000153485. [DOI] [PubMed] [Google Scholar]
- Fagerhol M. K., Cox D. W. The Pi polymorphism: genetic, biochemical, and clinical aspects of human alpha 1-antitrypsin. Adv Hum Genet. 1981;11:1-62, 371-2. [PubMed] [Google Scholar]
- Frants R. R., Eriksson A. W. Reliable classification of six Pi M subtypes by separator isoelectric focusing. Hum Hered. 1978;28(3):201–209. doi: 10.1159/000152959. [DOI] [PubMed] [Google Scholar]
- Genz T., Martin J. P., Cleve H. Classification of alpha1-antitrypsin (Pi) phenotypes by isoelectrofocusing. Distinction of six subtypes of the PiM phenotype. Hum Genet. 1977 Oct 14;38(3):325–332. doi: 10.1007/BF00402159. [DOI] [PubMed] [Google Scholar]
- Görg A., Postel W., Weser J., Patutschnick W., Cleve H. Improved resolution of PI (alpha 1-antitrypsin) phenotypes by a large-scale immobilized pH gradient. Am J Hum Genet. 1985 Sep;37(5):922–930. [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Travis J. Structural evidence for methionine at the reactive site of human alpha-1-proteinase inhibitor. J Biol Chem. 1978 Oct 25;253(20):7142–7144. [PubMed] [Google Scholar]
- Kueppers F., Christopherson M. J. Alpha1-antitrypsin: further genetic heterogeneity revealed by isoelectric focusing. Am J Hum Genet. 1978 Jul;30(4):359–365. [PMC free article] [PubMed] [Google Scholar]
- Kurachi K., Chandra T., Degen S. J., White T. T., Marchioro T. L., Woo S. L., Davie E. W. Cloning and sequence of cDNA coding for alpha 1-antitrypsin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6826–6830. doi: 10.1073/pnas.78.11.6826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Tiemeier D., Enquist L. EK2 derivatives of bacteriophage lambda useful in the cloning of DNA from higher organisms: the lambdagtWES system. Science. 1977 Apr 8;196(4286):175–177. doi: 10.1126/science.322278. [DOI] [PubMed] [Google Scholar]
- Leicht M., Long G. L., Chandra T., Kurachi K., Kidd V. J., Mace M., Jr, Davie E. W., Woo S. L. Sequence homology and structural comparison between the chromosomal human alpha 1-antitrypsin and chicken ovalbumin genes. Nature. 1982 Jun 24;297(5868):655–659. doi: 10.1038/297655a0. [DOI] [PubMed] [Google Scholar]
- Loebermann H., Tokuoka R., Deisenhofer J., Huber R. Human alpha 1-proteinase inhibitor. Crystal structure analysis of two crystal modifications, molecular model and preliminary analysis of the implications for function. J Mol Biol. 1984 Aug 15;177(3):531–557. [PubMed] [Google Scholar]
- Long G. L., Chandra T., Woo S. L., Davie E. W., Kurachi K. Complete sequence of the cDNA for human alpha 1-antitrypsin and the gene for the S variant. Biochemistry. 1984 Oct 9;23(21):4828–4837. doi: 10.1021/bi00316a003. [DOI] [PubMed] [Google Scholar]
- Nukiwa T., Brantly M., Garver R., Paul L., Courtney M., LeCocq J. P., Crystal R. G. Evaluation of "at risk" alpha 1-antitrypsin genotype SZ with synthetic oligonucleotide gene probes. J Clin Invest. 1986 Feb;77(2):528–537. doi: 10.1172/JCI112333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukiwa T., Brantly M., Ogushi F., Fells G., Satoh K., Stier L., Courtney M., Crystal R. G. Characterization of the M1(Ala213) type of alpha 1-antitrypsin, a newly recognized, common "normal" alpha 1-antitrypsin haplotype. Biochemistry. 1987 Aug 25;26(17):5259–5267. doi: 10.1021/bi00391a008. [DOI] [PubMed] [Google Scholar]
- Nukiwa T., Satoh K., Brantly M. L., Ogushi F., Fells G. A., Courtney M., Crystal R. G. Identification of a second mutation in the protein-coding sequence of the Z type alpha 1-antitrypsin gene. J Biol Chem. 1986 Dec 5;261(34):15989–15994. [PubMed] [Google Scholar]
- Nukiwa T., Takahashi H., Brantly M., Courtney M., Crystal R. G. alpha 1-Antitrypsin nullGranite Falls, a nonexpressing alpha 1-antitrypsin gene associated with a frameshift to stop mutation in a coding exon. J Biol Chem. 1987 Sep 5;262(25):11999–12004. [PubMed] [Google Scholar]
- Ogushi F., Fells G. A., Hubbard R. C., Straus S. D., Crystal R. G. Z-type alpha 1-antitrypsin is less competent than M1-type alpha 1-antitrypsin as an inhibitor of neutrophil elastase. J Clin Invest. 1987 Nov;80(5):1366–1374. doi: 10.1172/JCI113214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlino E., Cortese R., Ciliberto G. The human alpha 1-antitrypsin gene is transcribed from two different promoters in macrophages and hepatocytes. EMBO J. 1987 Sep;6(9):2767–2771. doi: 10.1002/j.1460-2075.1987.tb02571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin M., Watson M., Kidd V., Woo S. L., Breg W. R., Ruddle F. H. Regional location of alpha 1-antichymotrypsin and alpha 1-antitrypsin genes on human chromosome 14. Somat Cell Mol Genet. 1986 Mar;12(2):209–214. doi: 10.1007/BF01560668. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rogers J., Kalsheker N., Wallis S., Speer A., Coutelle C. H., Woods D., Humphries S. E. The isolation of a clone for human alpha 1-antitrypsin and the detection of alpha 1-antitrypsin in mRNA from liver and leukocytes. Biochem Biophys Res Commun. 1983 Oct 31;116(2):375–382. doi: 10.1016/0006-291x(83)90532-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg S., Barr P. J., Najarian R. C., Hallewell R. A. Synthesis in yeast of a functional oxidation-resistant mutant of human alpha-antitrypsin. Nature. 1984 Nov 1;312(5989):77–80. doi: 10.1038/312077a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen R. F., Li Y., Sifers R. N., Wang H., Hardick C., Tsai S. Y., Woo S. L. Tissue-specific expression of the human alpha 1-antitrypsin gene is controlled by multiple cis-regulatory elements. Nucleic Acids Res. 1987 Oct 26;15(20):8399–8415. doi: 10.1093/nar/15.20.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Travis J., Salvesen G. S. Human plasma proteinase inhibitors. Annu Rev Biochem. 1983;52:655–709. doi: 10.1146/annurev.bi.52.070183.003255. [DOI] [PubMed] [Google Scholar]
- Wewers M. D., Casolaro M. A., Sellers S. E., Swayze S. C., McPhaul K. M., Wittes J. T., Crystal R. G. Replacement therapy for alpha 1-antitrypsin deficiency associated with emphysema. N Engl J Med. 1987 Apr 23;316(17):1055–1062. doi: 10.1056/NEJM198704233161704. [DOI] [PubMed] [Google Scholar]
- Yoshida A., Chillar R., Taylor J. C. An alpha 1-antitrypsin variant, Pi B Alhambra (Lys to Asp, Glu to Asp), with rapid anodal electrophoretic mobility. Am J Hum Genet. 1979 Sep;31(5):555–563. [PMC free article] [PubMed] [Google Scholar]
- Yoshida A., Taylor J. C., van den Brock W. G. Structural difference between the normal PiM1 and the common PiM2 variant of human alpha 1-antitrypsin. Am J Hum Genet. 1979 Sep;31(5):564–568. [PMC free article] [PubMed] [Google Scholar]