Abstract
Apolipoprotein AI (apo AI) is the major protein of high-density lipoprotein (HDL). Using radioimmunoassay, we measured plasma apo AI levels in 1,880 individuals in 283 pedigrees randomly selected from the population with respect to disease status and risk factors for coronary artery disease. Apo AI levels were first adjusted for date of assay (6.8% of apo AI variation) and then adjusted for variability in age and body mass index (an additional 6.6%, 20.4%, and 23.0% of apo AI variations for males, females not using exogenous hormones, and females using exogenous hormones, respectively). A mixture of two normal distributions fit the adjusted data better than did a single normal distribution. Genetic and environmental models that could explain the mixture of normal distributions were investigated using complex segregation analysis. Heterogeneous etiologies for individual differences in adjusted apo AI levels were suggested by the data in the 283 pedigrees. In a subset of 126 pedigrees, there is evidence for the major effect of a nontransmitted environmental factor that explains the mixture of distributions as well as polygenic loci that influence apo AI levels within each distribution. The environmental factor and polygenic loci account for 32% and 65% of the adjusted variation, respectively. In the other 157 pedigrees there is strong support for a single locus with a major effect that accounts for 27% of the adjusted variation. The effect of the polygenic loci is not different from zero in these 157 pedigrees. This is the first study to present evidence for the segregation of a single unmeasured locus with a major effect on levels of apo AI in a population-based sample of pedigrees.
Full text
PDF



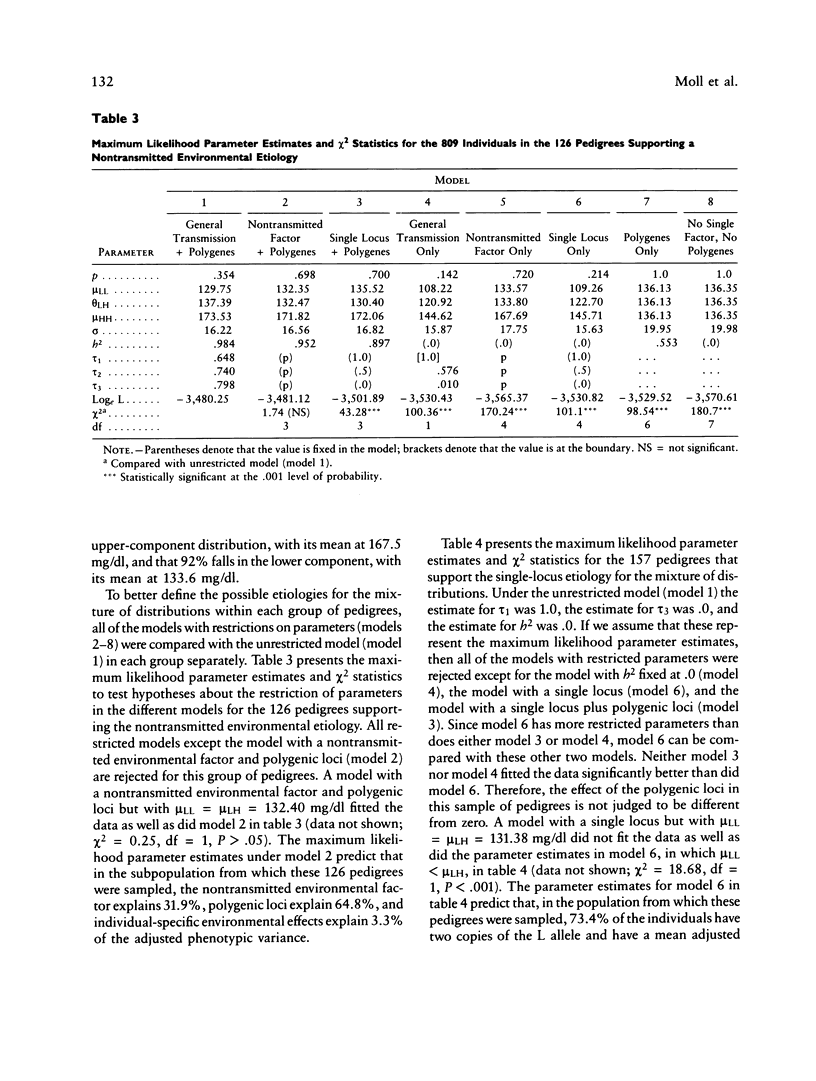
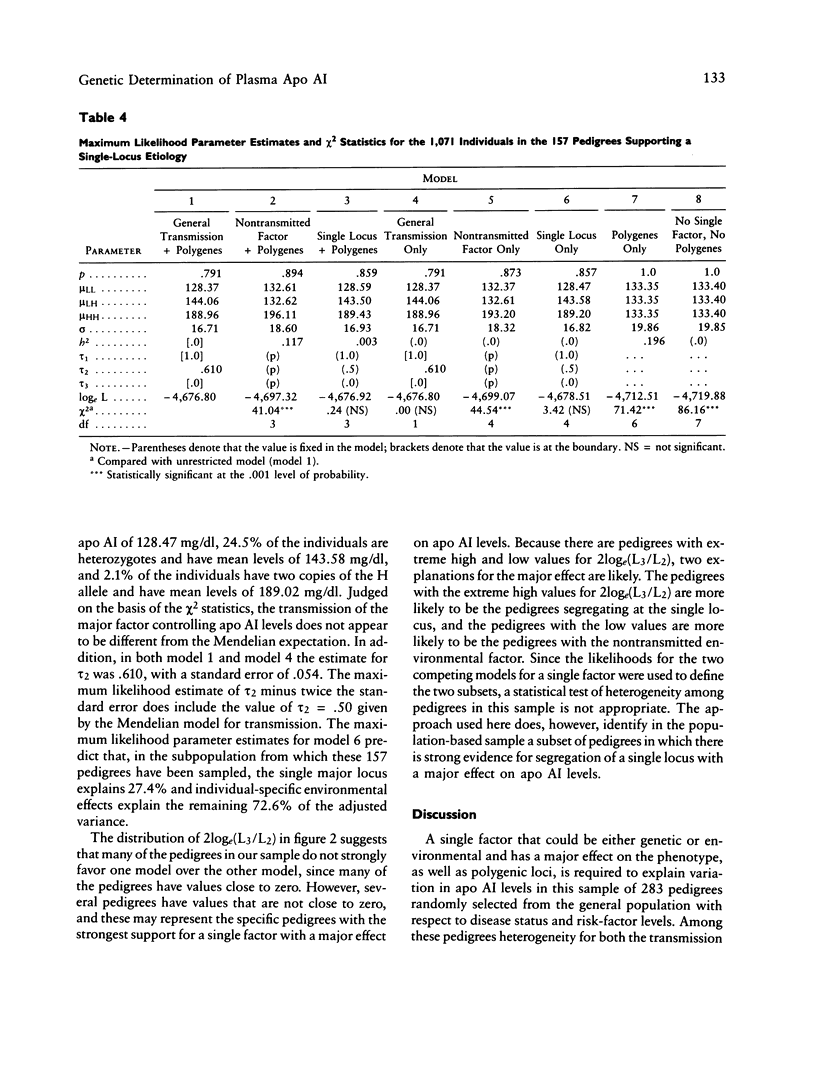






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos C. I., Elston R. C., Srinivasan S. R., Wilson A. F., Cresanta J. L., Ward L. J., Berenson G. S. Linkage and segregation analyses of apolipoproteins A1 and B, and lipoprotein cholesterol levels in a large pedigree with excess coronary heart disease: the Bogalusa Heart Study. Genet Epidemiol. 1987;4(2):115–128. doi: 10.1002/gepi.1370040206. [DOI] [PubMed] [Google Scholar]
- Au Y. P., Hallaway B. J., Kottke B. A. Use of a quality-control plasma sample to decrease interassay variation in radioimmunoassays of apolipoprotein A-I. Clin Chem. 1986 Jul;32(7):1394–1397. [PubMed] [Google Scholar]
- Beaty T. H., Boughman J. A. Problems in detecting etiological heterogeneity in genetic disease illustrated with retinitis pigmentosa. Am J Med Genet. 1986 Jul;24(3):493–504. doi: 10.1002/ajmg.1320240312. [DOI] [PubMed] [Google Scholar]
- Beaty T. H. Discriminating among single locus models using small pedigrees. Am J Med Genet. 1980;6(3):229–240. doi: 10.1002/ajmg.1320060307. [DOI] [PubMed] [Google Scholar]
- Berg A., Frey I., Keul J. Apolipoprotein profile in healthy males and its relation to maximum aerobic capacity (MAC). Clin Chim Acta. 1986 Dec 15;161(2):165–171. doi: 10.1016/0009-8981(86)90210-x. [DOI] [PubMed] [Google Scholar]
- Berg K. Twin studies of coronary heart disease and its risk factors. Acta Genet Med Gemellol (Roma) 1984;33(3):349–361. doi: 10.1017/s0001566000005808. [DOI] [PubMed] [Google Scholar]
- Boerwinkle E., Chakraborty R., Sing C. F. The use of measured genotype information in the analysis of quantitative phenotypes in man. I. Models and analytical methods. Ann Hum Genet. 1986 May;50(Pt 2):181–194. doi: 10.1111/j.1469-1809.1986.tb01037.x. [DOI] [PubMed] [Google Scholar]
- Boerwinkle E., Sing C. F. The use of measured genotype information in the analysis of quantitative phenotypes in man. III. Simultaneous estimation of the frequencies and effects of the apolipoprotein E polymorphism and residual polygenetic effects on cholesterol, betalipoprotein and triglyceride levels. Ann Hum Genet. 1987 Jul;51(Pt 3):211–226. doi: 10.1111/j.1469-1809.1987.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Boerwinkle E., Utermann G. Simultaneous effects of the apolipoprotein E polymorphism on apolipoprotein E, apolipoprotein B, and cholesterol metabolism. Am J Hum Genet. 1988 Jan;42(1):104–112. [PMC free article] [PubMed] [Google Scholar]
- Boerwinkle E., Visvikis S., Welsh D., Steinmetz J., Hanash S. M., Sing C. F. The use of measured genotype information in the analysis of quantitative phenotypes in man. II. The role of the apolipoprotein E polymorphism in determining levels, variability, and covariability of cholesterol, betalipoprotein, and triglycerides in a sample of unrelated individuals. Am J Med Genet. 1987 Jul;27(3):567–582. doi: 10.1002/ajmg.1320270310. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Fairwell T., LaRue A., Ronan R., Houser A., Bronzert T. J. The amino acid sequence of human APOA-I, an apolipoprotein isolated from high density lipoproteins. Biochem Biophys Res Commun. 1978 Feb 14;80(3):623–630. doi: 10.1016/0006-291x(78)91614-5. [DOI] [PubMed] [Google Scholar]
- Bruns G. A., Karathanasis S. K., Breslow J. L. Human apolipoprotein A-I--C-III gene complex is located on chromosome 11. Arteriosclerosis. 1984 Mar-Apr;4(2):97–102. doi: 10.1161/01.atv.4.2.97. [DOI] [PubMed] [Google Scholar]
- Brunzell J. D., Sniderman A. D., Albers J. J., Kwiterovich P. O., Jr Apoproteins B and A-I and coronary artery disease in humans. Arteriosclerosis. 1984 Mar-Apr;4(2):79–83. doi: 10.1161/01.atv.4.2.79. [DOI] [PubMed] [Google Scholar]
- Cheung P., Kao F. T., Law M. L., Jones C., Puck T. T., Chan L. Localization of the structural gene for human apolipoprotein A-I on the long arm of human chromosome 11. Proc Natl Acad Sci U S A. 1984 Jan;81(2):508–511. doi: 10.1073/pnas.81.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon J., Gregg R. E., Sing C. F. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988 Jan-Feb;8(1):1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- Deeb S., Failor A., Brown B. G., Brunzell J. D., Albers J. J., Motulsky A. G. Molecular genetics of apolipoproteins and coronary heart disease. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):403–409. doi: 10.1101/sqb.1986.051.01.048. [DOI] [PubMed] [Google Scholar]
- Demenais F., Lathrop M., Lalouel J. M. Robustness and power of the unified model in the analysis of quantitative measurements. Am J Hum Genet. 1986 Feb;38(2):228–234. [PMC free article] [PubMed] [Google Scholar]
- Elston R. C., Stewart J. A general model for the genetic analysis of pedigree data. Hum Hered. 1971;21(6):523–542. doi: 10.1159/000152448. [DOI] [PubMed] [Google Scholar]
- Franceschini G., Sirtori C. R., Capurso A., 2nd, Weisgraber K. H., Mahley R. W. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest. 1980 Nov;66(5):892–900. doi: 10.1172/JCI109956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman D. S., Srinivasan S. R., Shear C. L., Franklin F. A., Webber L. S., Berenson G. S. The relation of apolipoproteins A-I and B in children to parental myocardial infarction. N Engl J Med. 1986 Sep 18;315(12):721–726. doi: 10.1056/NEJM198609183151202. [DOI] [PubMed] [Google Scholar]
- Haffner S. M., Applebaum-Bowden D., Wahl P. W., Hoover J. J., Warnick G. R., Albers J. J., Hazzard W. R. Epidemiological correlates of high density lipoprotein subfractions, apolipoproteins A-I, A-II, and D, and lecithin cholesterol acyltransferase. Effects of smoking, alcohol, and adiposity. Arteriosclerosis. 1985 Mar-Apr;5(2):169–177. doi: 10.1161/01.atv.5.2.169. [DOI] [PubMed] [Google Scholar]
- Hamsten A., Iselius L., Dahlén G., de Faire U. Genetic and cultural inheritance of serum lipids, low and high density lipoprotein cholesterol and serum apolipoproteins A-I, A-II and B. Atherosclerosis. 1986 Jun;60(3):199–208. doi: 10.1016/0021-9150(86)90166-8. [DOI] [PubMed] [Google Scholar]
- Hartung G. H., Reeves R. S., Foreyt J. P., Patsch W., Gotto A. M., Jr Effect of alcohol intake and exercise on plasma high-density lipoprotein cholesterol subfractions and apolipoprotein A-I in women. Am J Cardiol. 1986 Jul 1;58(1):148–151. doi: 10.1016/0002-9149(86)90259-6. [DOI] [PubMed] [Google Scholar]
- Hasstedt S. J. A mixed-model likelihood approximation on large pedigrees. Comput Biomed Res. 1982 Jun;15(3):295–307. doi: 10.1016/0010-4809(82)90064-7. [DOI] [PubMed] [Google Scholar]
- Hasstedt S. J., Albers J. J., Cheung M. C., Jorde L. B., Wilson D. E., Edwards C. Q., Cannon W. N., Ash K. O., Williams R. R. The inheritance of high density lipoprotein cholesterol and apolipoproteins A-I and A-II. Atherosclerosis. 1984 Apr;51(1):21–29. doi: 10.1016/0021-9150(84)90141-2. [DOI] [PubMed] [Google Scholar]
- Hegele R. A., Breslow J. L. Apolipoprotein genetic variation in the assessment of atherosclerosis susceptibility. Genet Epidemiol. 1987;4(3):163–184. doi: 10.1002/gepi.1370040302. [DOI] [PubMed] [Google Scholar]
- Karathanasis S. K. Apolipoprotein multigene family: tandem organization of human apolipoprotein AI, CIII, and AIV genes. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6374–6378. doi: 10.1073/pnas.82.19.6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karathanasis S. K., Norum R. A., Zannis V. I., Breslow J. L. An inherited polymorphism in the human apolipoprotein A-I gene locus related to the development of atherosclerosis. Nature. 1983 Feb 24;301(5902):718–720. doi: 10.1038/301718a0. [DOI] [PubMed] [Google Scholar]
- Kessling A. M., Rajput-Wiliams J., Bainton D., Scott J., Miller N. E., Baker I., Humphries S. E. DNA polymorphisms of the apolipoprotein AII and AI-CIII-AIV genes: a study in men selected for differences in high-density-lipoprotein cholesterol concentration. Am J Hum Genet. 1988 Mar;42(3):458–467. [PMC free article] [PubMed] [Google Scholar]
- Koizumi J., Mabuchi H., Yoshimura A., Michishita I., Takeda M., Itoh H., Sakai Y., Sakai T., Ueda K., Takeda R. Deficiency of serum cholesteryl-ester transfer activity in patients with familial hyperalphalipoproteinaemia. Atherosclerosis. 1985 Dec;58(1-3):175–186. doi: 10.1016/0021-9150(85)90064-4. [DOI] [PubMed] [Google Scholar]
- Koren E., Puchois P., Alaupovic P., Fesmire J., Kandoussi A., Fruchart J. C. Quantification of two different types of apolipoprotein A-I containing lipoprotein particles in plasma by enzyme-linked differential-antibody immunosorbent assay. Clin Chem. 1987 Jan;33(1):38–43. [PubMed] [Google Scholar]
- Kottke B. A., Zinsmeister A. R., Holmes D. R., Jr, Kneller R. W., Hallaway B. J., Mao S. J. Apolipoproteins and coronary artery disease. Mayo Clin Proc. 1986 May;61(5):313–320. doi: 10.1016/s0025-6196(12)61947-8. [DOI] [PubMed] [Google Scholar]
- Kuusi T., Kesäniemi Y. A., Vuoristo M., Miettinen T. A., Koskenvuo M. Inheritance of high density lipoprotein and lipoprotein lipase and hepatic lipase activity. Arteriosclerosis. 1987 Jul-Aug;7(4):421–425. doi: 10.1161/01.atv.7.4.421. [DOI] [PubMed] [Google Scholar]
- Lalouel J. M., Rao D. C., Morton N. E., Elston R. C. A unified model for complex segregation analysis. Am J Hum Genet. 1983 Sep;35(5):816–826. [PMC free article] [PubMed] [Google Scholar]
- Lusis A. J. Genetic factors affecting blood lipoproteins: the candidate gene approach. J Lipid Res. 1988 Apr;29(4):397–429. [PubMed] [Google Scholar]
- MacLean C. J., Morton N. E., Lew R. Analysis of family resemblance. IV. Operational characteristics of segregation analysis. Am J Hum Genet. 1975 May;27(3):365–384. [PMC free article] [PubMed] [Google Scholar]
- Maciejko J. J., Holmes D. R., Kottke B. A., Zinsmeister A. R., Dinh D. M., Mao S. J. Apolipoprotein A-I as a marker of angiographically assessed coronary-artery disease. N Engl J Med. 1983 Aug 18;309(7):385–389. doi: 10.1056/NEJM198308183090701. [DOI] [PubMed] [Google Scholar]
- Maciejko J. J., Mao S. J. Radioimmunoassay of apolipoprotein A-i. application of a non-ionic detergent (Tween-20) and solid-phase staphylococcus. Clin Chem. 1982 Jan;28(1):199–204. [PubMed] [Google Scholar]
- Maclean C. J., Morton N. E., Elston R. C., Yee S. Skewness in commingled distributions. Biometrics. 1976 Sep;32(3):695–699. [PubMed] [Google Scholar]
- Menzel H. J., Assmann G., Rall S. C., Jr, Weisgraber K. H., Mahley R. W. Human apolipoprotein A-I polymorphism. Identification of amino acid substitutions in three electrophoretic variants of the Münster-3 type. J Biol Chem. 1984 Mar 10;259(5):3070–3076. [PubMed] [Google Scholar]
- Moll P. P., Sing C. F., Williams R. R., Mao S. J., Kottke B. A. The genetic determination of plasma apolipoprotein A-I levels measured by radioimmunoassay: a study of high-risk pedigrees. Am J Hum Genet. 1986 Mar;38(3):361–372. [PMC free article] [PubMed] [Google Scholar]
- Neel J. V. A revised estimate of the amount of genetic variation in human proteins: implications for the distribution of DNA polymorphisms. Am J Hum Genet. 1984 Sep;36(5):1135–1148. [PMC free article] [PubMed] [Google Scholar]
- Ordovas J. M., Schaefer E. J., Salem D., Ward R. H., Glueck C. J., Vergani C., Wilson P. W., Karathanasis S. K. Apolipoprotein A-I gene polymorphism associated with premature coronary artery disease and familial hypoalphalipoproteinemia. N Engl J Med. 1986 Mar 13;314(11):671–677. doi: 10.1056/NEJM198603133141102. [DOI] [PubMed] [Google Scholar]
- Patsch J. R., Gotto A. M., Jr, Olivercrona T., Eisenberg S. Formation of high density lipoprotein2-like particles during lipolysis of very low density lipoproteins in vitro. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4519–4523. doi: 10.1073/pnas.75.9.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonfeld G., Lees R. S., George P. K., Pfleger B. Assay of total plasma apolipoprotein B concentration in human subjects. J Clin Invest. 1974 May;53(5):1458–1467. doi: 10.1172/JCI107694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing C. F., Davignon J. Role of the apolipoprotein E polymorphism in determining normal plasma lipid and lipoprotein variation. Am J Hum Genet. 1985 Mar;37(2):268–285. [PMC free article] [PubMed] [Google Scholar]
- Sistonen P., Ehnholm C. On the heritability of serum high density lipoprotein in twins. Am J Hum Genet. 1980 Jan;32(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Stein O., Fainaru M., Stein Y. The role of lysophosphatidylcholine and apolipoprotein A in the cholesterol-removing capacity of lipoprotein-deficient serum in tissue culture. Biochim Biophys Acta. 1979 Sep 28;574(3):495–504. doi: 10.1016/0005-2760(79)90246-7. [DOI] [PubMed] [Google Scholar]
- Utermann G., Steinmetz A., Paetzold R., Wilk J., Feussner G., Kaffarnik H., Mueller-Eckhardt C., Seidel D., Vogelberg K. H., Zimmer F. Apolipoprotein AIMarburg: studies on two kindreds with a mutant of human apolipoprotein AI. Hum Genet. 1982;61(4):329–337. doi: 10.1007/BF00276597. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Anderson R. A. Blood lipids, lipid-related measures, and the risk of atherosclerotic cardiovascular disease. Epidemiol Rev. 1987;9:95–119. doi: 10.1093/oxfordjournals.epirev.a036310. [DOI] [PubMed] [Google Scholar]


