Abstract
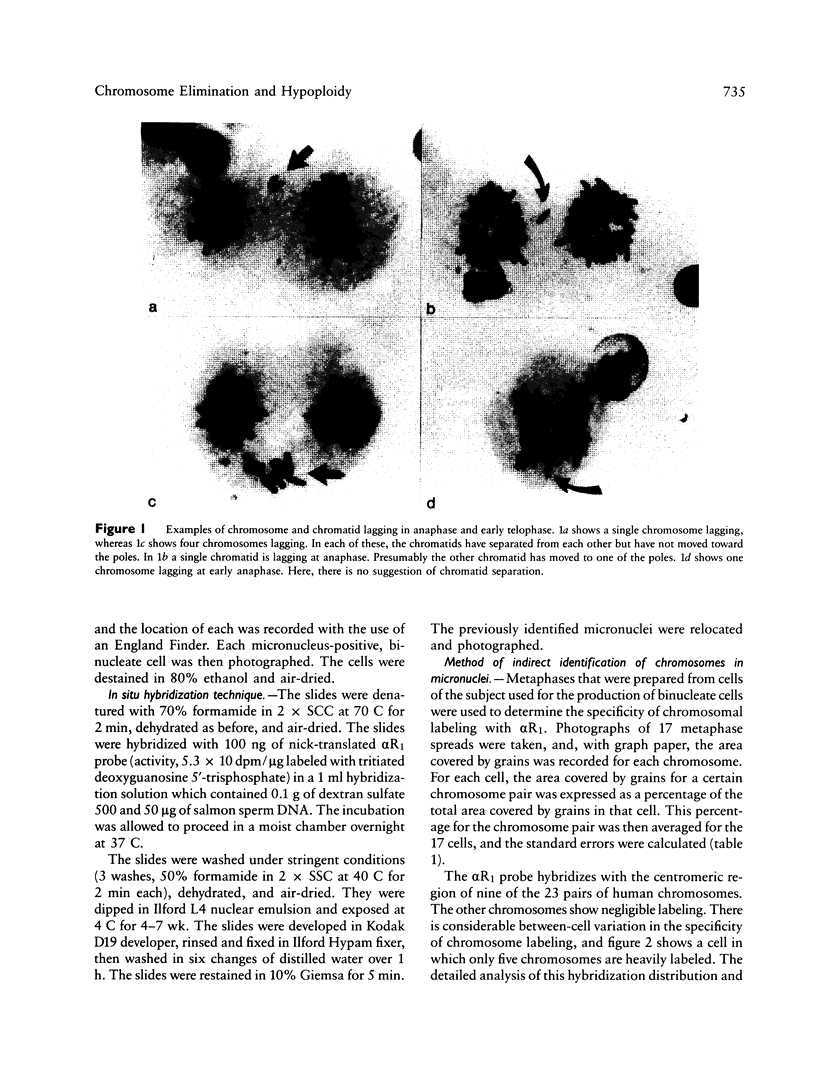
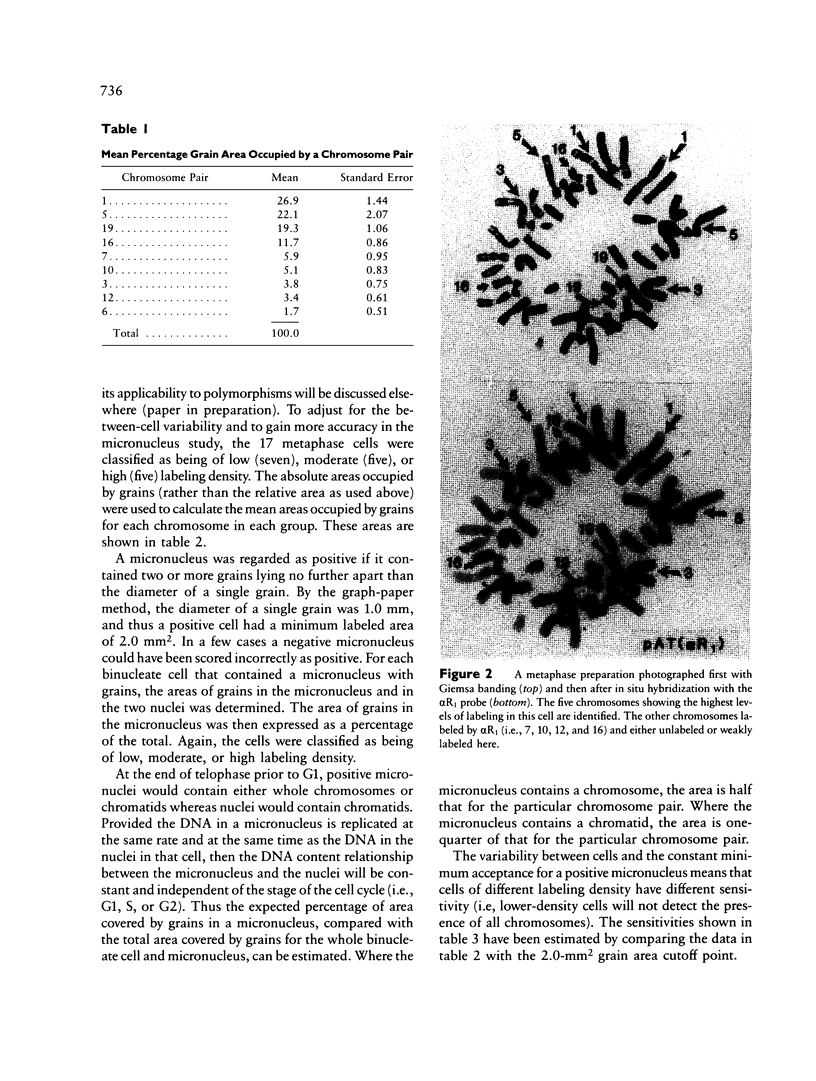
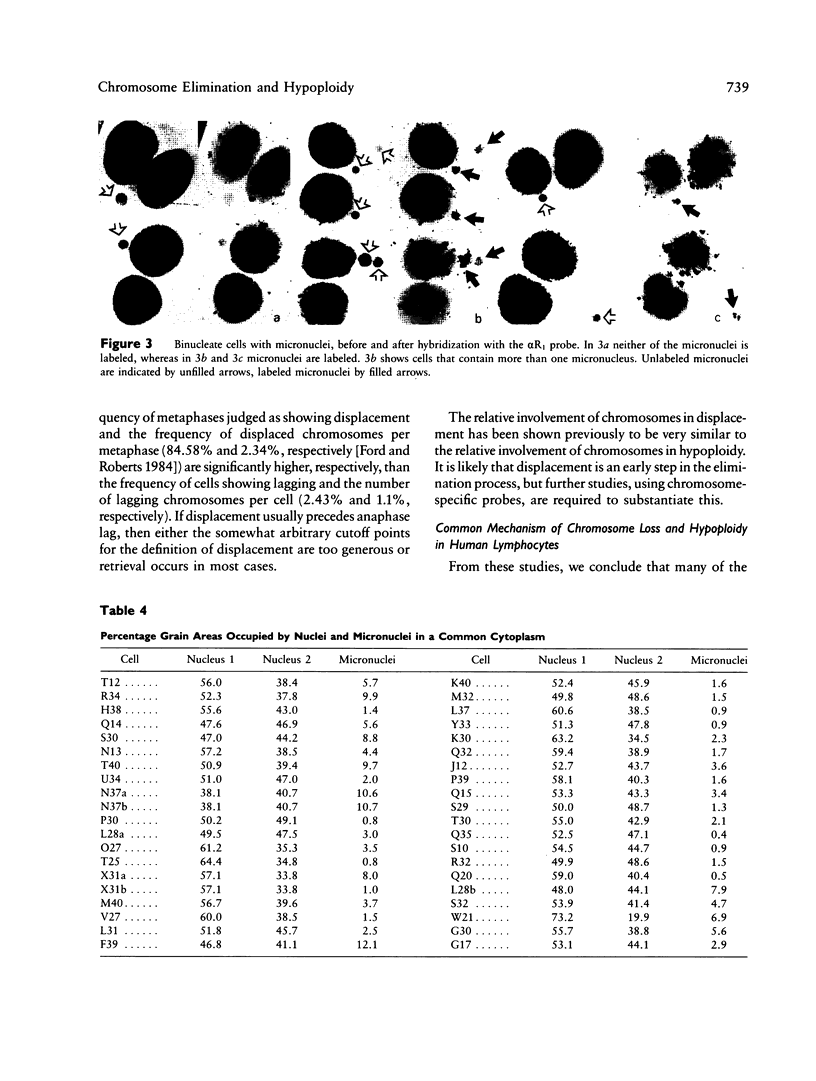
An excess of hypoploid cells has repeatedly been reported in studies of aneuploidy and has often been attributed to technical artifact. We have examined at least 200 anaphase or early-telophase cells from each of 28 normal women and found that chromosome or chromatid lagging occurs in an average of 2.43% of cells. In a separate study, we have examined the frequency of micronuclei in cytochalasin B-arrested, binucleate cells and shown that a similar frequency of cells (1.6%) contain one or more micronuclei. Using in situ hybridization of an alpha centromeric probe (alpha R1), which hybridizes to 9 of the 22 human autosomes, we were able to infer that most, if not all, of the micronuclei contain whole chromosomes or chromatids. Since the loss of a chromosome by lagging will induce hypoploid daughter nuclei (two where a chromosome is lost and one where a chromatid is lost), we conclude that lagging is a major mechanism for chromosome loss in human lymphocyte cultures. This loss occurs in the cells of normal individuals under control conditions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T., Fox D. P., Robertson F. W., Bullock I. Non-random chromosome loss in PHA-stimulated lymphocytes from normal individuals. Mutat Res. 1983 Dec;122(3-4):403–406. doi: 10.1016/0165-7992(83)90027-1. [DOI] [PubMed] [Google Scholar]
- Correll A. T., Ford J. H. Chromosome flexion: potential for assessing the state of spindle assembly. Mutat Res. 1987 Feb;190(2):137–143. doi: 10.1016/0165-7992(87)90045-5. [DOI] [PubMed] [Google Scholar]
- Fenech M., Morley A. Solutions to the kinetic problem in the micronucleus assay. Cytobios. 1985;43(172-173):233–246. [PubMed] [Google Scholar]
- Fitzgerald P. H., McEwan C. M. Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum Genet. 1977 Dec 23;39(3):329–337. doi: 10.1007/BF00295428. [DOI] [PubMed] [Google Scholar]
- Ford C. E. Nondisjunction. Hum Genet Suppl. 1981;2:103–143. doi: 10.1007/978-3-642-68006-9_9. [DOI] [PubMed] [Google Scholar]
- Ford J. H., Congedi M. M. Rapid induction of anaphase in competent cells by hypotonic treatment. Cytobios. 1987;51(206-207):183–192. [PubMed] [Google Scholar]
- Ford J. H., Lester P. Factors affecting the displacement of human chromosomes from the metaphase plate. Cytogenet Cell Genet. 1982;33(4):327–332. doi: 10.1159/000131779. [DOI] [PubMed] [Google Scholar]
- Ford J. H., Roberts C. G. Chromosome displacement and spindle tubule polymerization. 2. Effect of alterations of extracellular calcium on displacement parameters. Cytobios. 1984;39(154):81–88. [PubMed] [Google Scholar]
- Ford J. H., Roberts C. Displacement of chromosomes in mitosis: a technique for assessing differential chromosome error. Cytogenet Cell Genet. 1983;36(3):537–541. doi: 10.1159/000131969. [DOI] [PubMed] [Google Scholar]
- Ridler M. A., Smith G. F. The response of human cultured lymphocytes to cytochalasin B. J Cell Sci. 1968 Dec;3(4):595–602. doi: 10.1242/jcs.3.4.595. [DOI] [PubMed] [Google Scholar]
- Viaggi S., Bonatti S., Abbondandolo A. New evidence for the presence of chromosomes in micronuclei of human and Chinese hamster cells. Mutagenesis. 1987 Sep;2(5):367–370. doi: 10.1093/mutage/2.5.367. [DOI] [PubMed] [Google Scholar]