Abstract
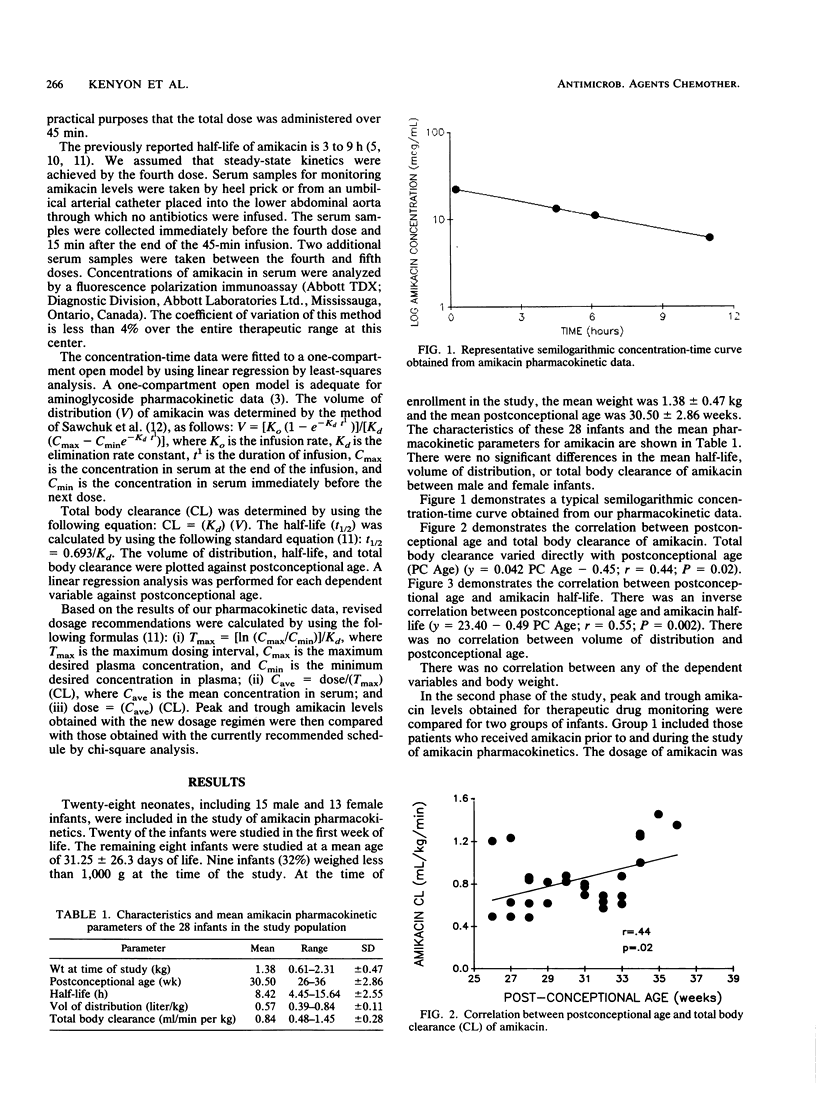
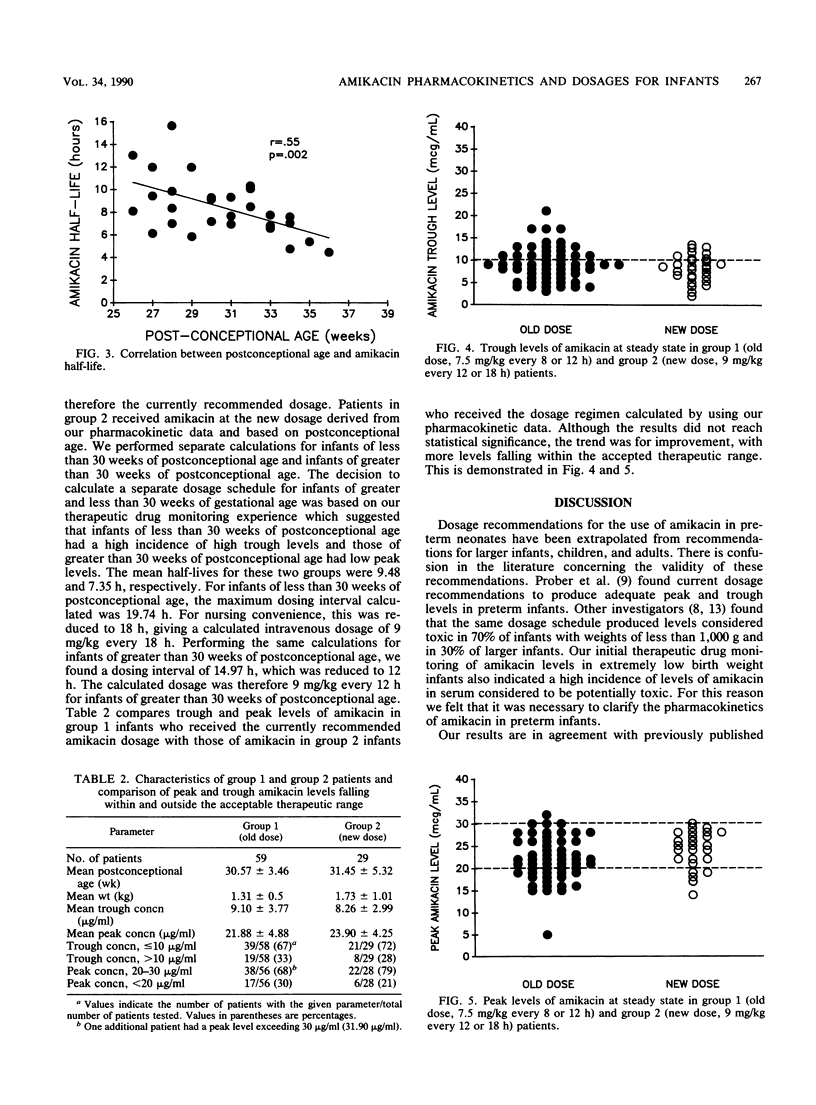
The pharmacokinetics of amikacin administered intravenously at currently recommended doses (7.5 mg/kg every 12 h for infants with less than 7 days of life; 7.5 mg/kg every 8 h for infants with greater than 7 days of life) were studied in 28 preterm infants weighing less than 2,500 g (mean +/- standard deviation, 1.38 +/- 0.47 kg; postconceptional age, 30.50 +/- 2.86 weeks). The medication was infused over 45 min. Trough and peak serum samples as well as two additional samples were taken at steady state. The results showed a statistically significant inverse relationship between half-life (8.42 +/- 2.55 h) and postconceptional age (P = 0.002) and a direct correlation between total body clearance (0.84 +/- 0.28 ml/min per kg) and postconceptional age (P = 0.02). These pharmacokinetic data were used to calculate a new dosage schedule for preterm infants. The derived intravenous dosage of amikacin for infants of less than 30 weeks of postconceptional age was 9 mg/kg every 18 h. For infants of greater than 30 weeks of postconceptional age, the dosage was 9 mg/kg every 12 h. Peak and trough levels of amikacin in serum that fell within the therapeutic range were compared by using the currently recommended dosage schedule and the dosage schedule derived from our pharmacokinetic data. There was a reduction in the number of peak and trough levels that fell outside the accepted therapeutic range which was not statistically significant. Extension of the dosing interval and a further increase in the dosage may result in further improvement. Based on these data, the current recommendations are inadequate for the preterm infant. Our derived dosage schedule improved but did not eliminate high trough and low peak levels of amikacin in all infants. The current recommendations should be adjusted for the preterm infant. Ongoing therapeutic drug monitoring is essential to tailor the amikacin dosage to the individual patient.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arant B. S., Jr Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr. 1978 May;92(5):705–712. doi: 10.1016/s0022-3476(78)80133-4. [DOI] [PubMed] [Google Scholar]
- Arant B. S., Jr Nonrenal factors influencing renal function during the perinatal period. Clin Perinatol. 1981 Jun;8(2):225–240. [PubMed] [Google Scholar]
- Howard J. B., McCracken G. H., Jr Pharmacological evaluation of amikacin in neonates. Antimicrob Agents Chemother. 1975 Jul;8(1):86–90. doi: 10.1128/aac.8.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J. B., McCraken G. H., Jr, Trujillo H., Mohs E. Amikacin in newborn infants: comparative pharmacology with kanamycin and clinical efficacy in 45 neonates with bacterial diseases. Antimicrob Agents Chemother. 1976 Aug;10(2):205–210. doi: 10.1128/aac.10.2.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips J. B., 3rd, Satterwhite C., Dworsky M. E., Cassady G. Recommended amikacin doses in newborns often produce excessive serum levels. Pediatr Pharmacol (New York) 1982;2(2):121–125. [PubMed] [Google Scholar]
- Philips J. B., Cassady G. Amikacin: pharmacology, indications and cautions for use, and dose recommendations. Semin Perinatol. 1982 Apr;6(2):166–171. [PubMed] [Google Scholar]
- Prober C. G., Yeager A. S., Arvin A. M. The effect of chronologic age on the serum concentrations of amikacin in sick term and premature infants. J Pediatr. 1981 Apr;98(4):636–640. doi: 10.1016/s0022-3476(81)80783-4. [DOI] [PubMed] [Google Scholar]
- Riff L., Schauf V. Use of aminoglycosides in the neonate. Semin Perinatol. 1982 Apr;6(2):155–165. [PubMed] [Google Scholar]
- Sawchuk R. J., Zaske D. E., Cipolle R. J., Wargin W. A., Strate R. G. Kinetic model for gentamicin dosing with the use of individual patient parameters. Clin Pharmacol Ther. 1977 Mar;21(3):362–369. doi: 10.1002/cpt1977213362. [DOI] [PubMed] [Google Scholar]
- Shulman S. T., Yogev R. Treatment of pediatric infections with amikacin as first-line aminoglycoside. Am J Med. 1985 Jul 15;79(1A):43–50. doi: 10.1016/0002-9343(85)90190-1. [DOI] [PubMed] [Google Scholar]
- Smith C. R., Maxwell R. R., Edwards C. Q., Rogers J. F., Lietman P. S. Nephrotoxicity induced by gentamicin and amikacin. Johns Hopkins Med J. 1978 Mar;142(3):85–90. [PubMed] [Google Scholar]
- Vogelstein B., Kowarski A., Lietman P. S. The pharmacokinetics of amikacin in children. J Pediatr. 1977 Aug;91(2):333–339. doi: 10.1016/s0022-3476(77)80847-0. [DOI] [PubMed] [Google Scholar]


