Abstract
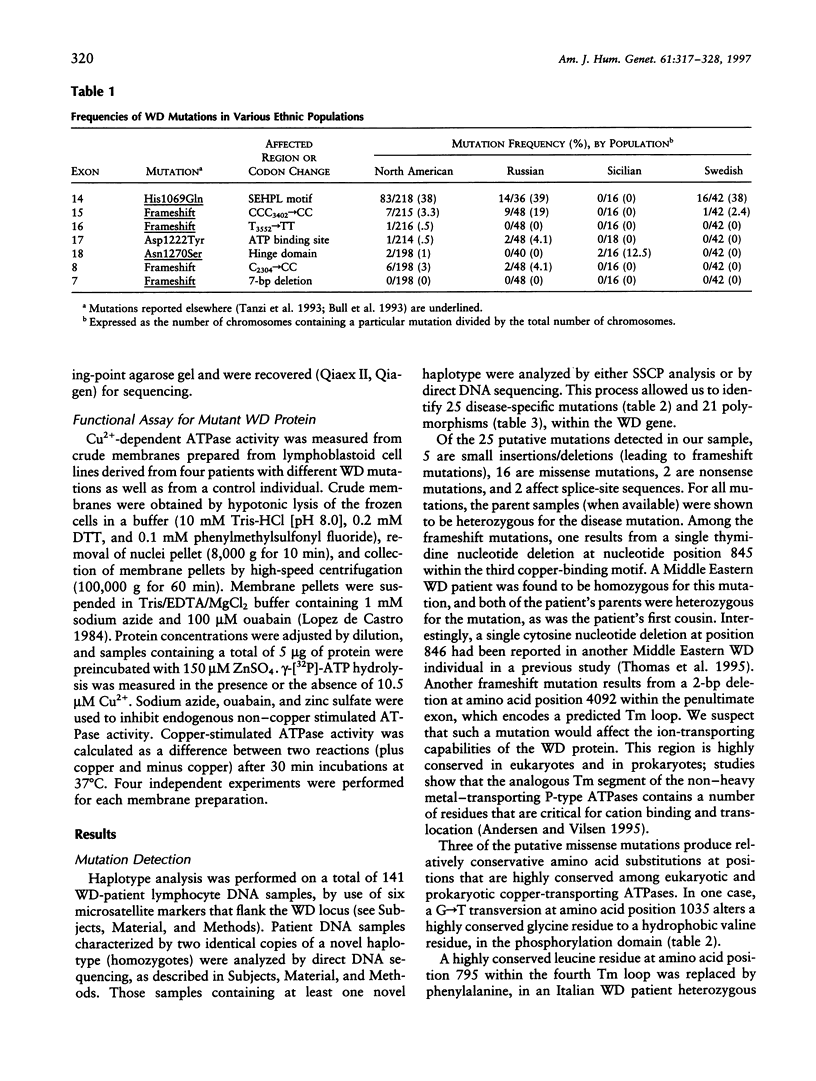
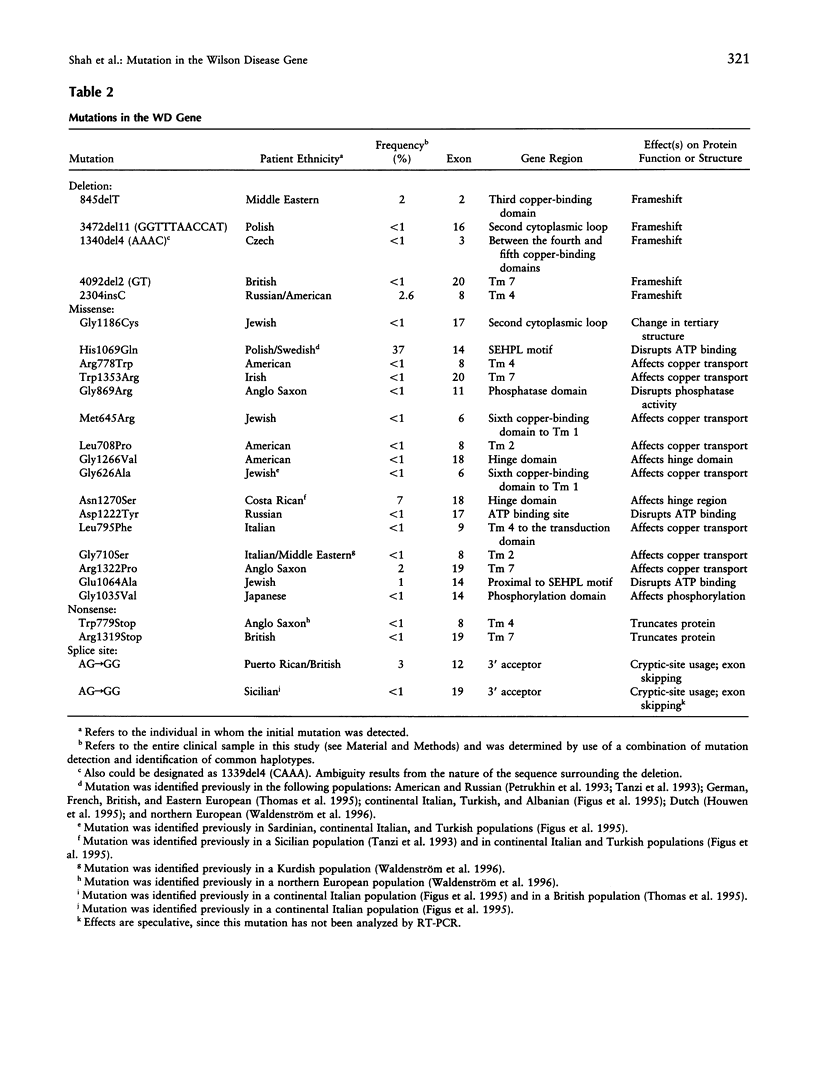
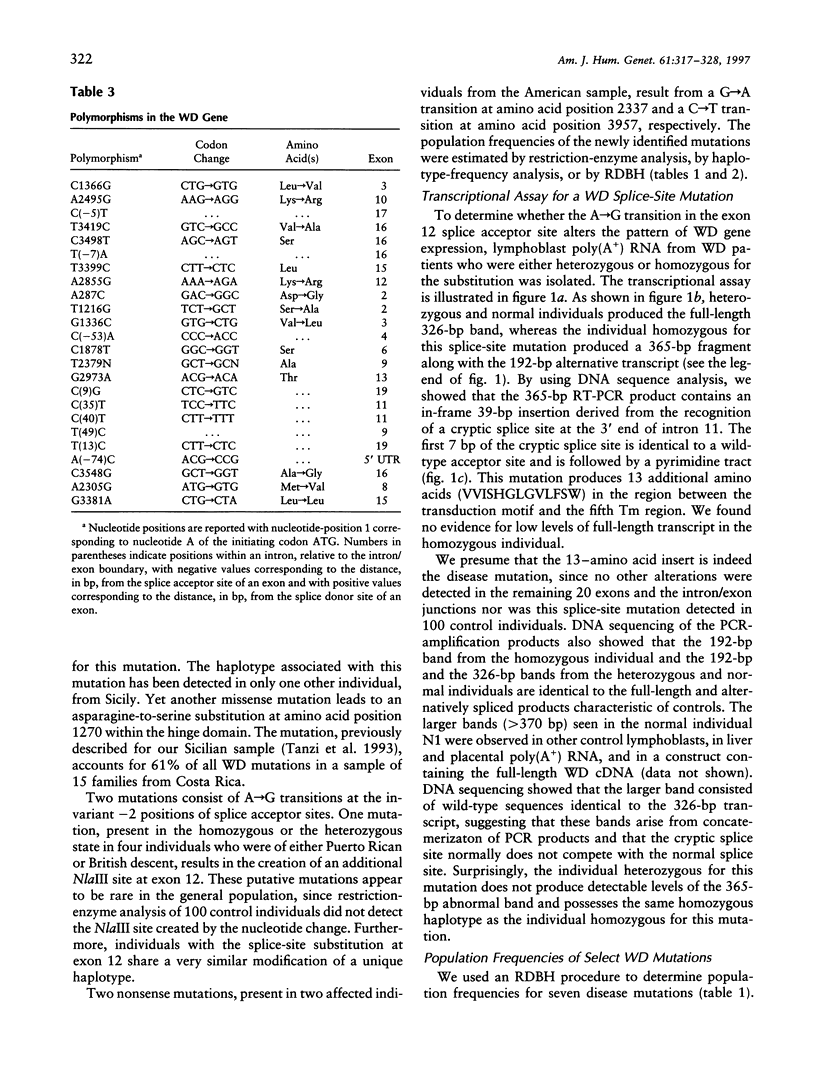
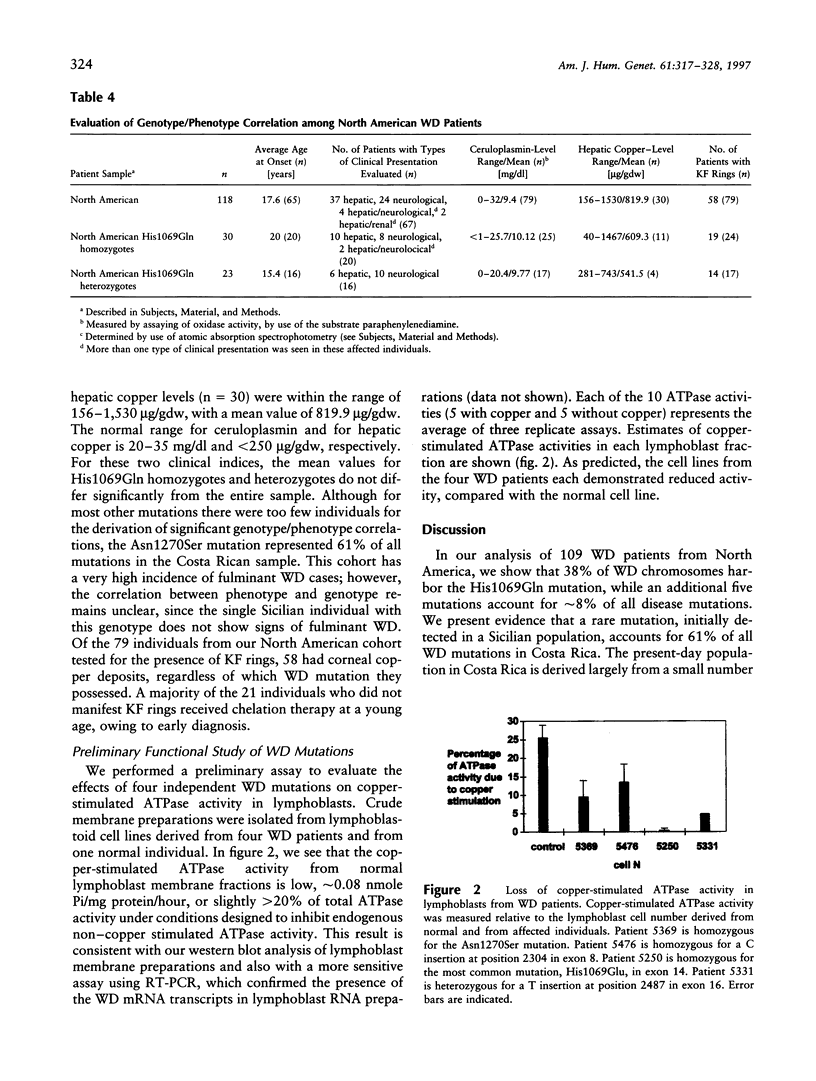
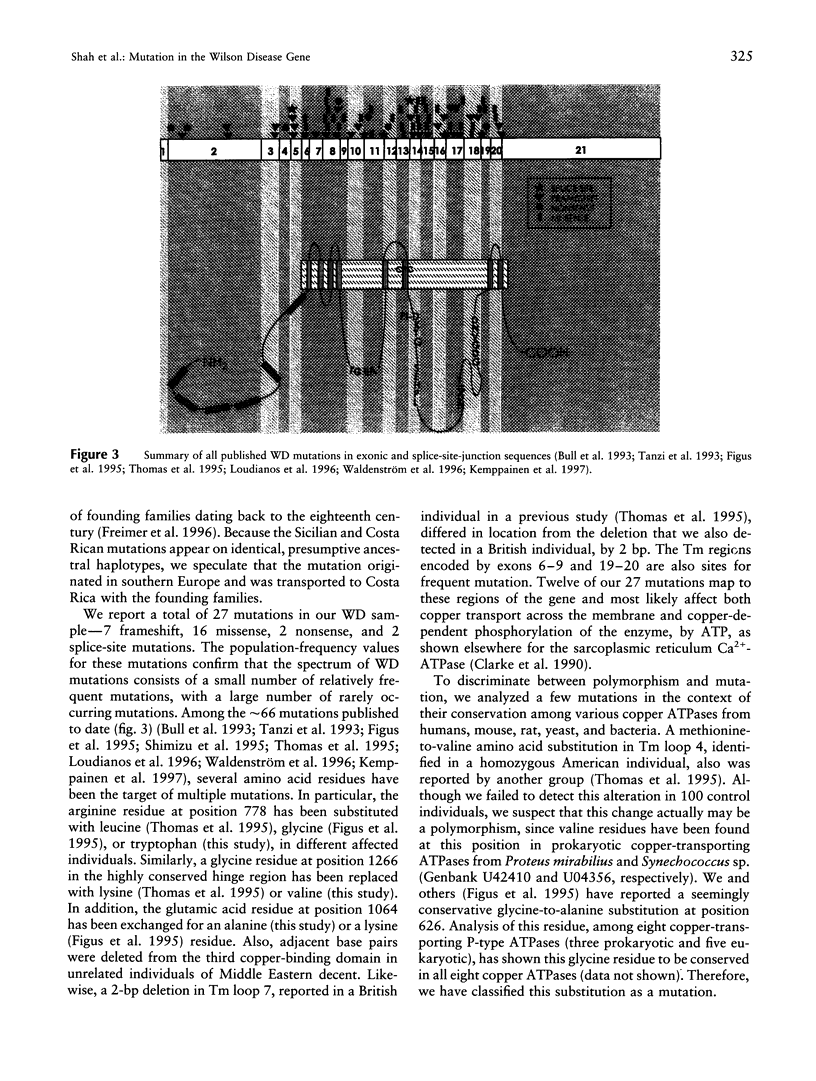
Wilson disease (WD) is an autosomal recessive disorder characterized by toxic accumulation of copper in the liver and subsequently in the brain and other organs. On the basis of sequence homology to known genes, the WD gene (ATP7B) appears to be a copper-transporting P-type ATPase. A search for ATP7B mutations in WD patients from five population samples, including 109 North American patients, revealed 27 distinct mutations, 18 of which are novel. A composite of published findings shows missense mutations in all exons-except in exons 1-5, which encode the six copper-binding motifs, and in exon 21, which spans the carboxy-terminus and the poly(A) tail. Over one-half of all WD mutations occur only rarely in any population sample. A splice-site mutation in exon 12 accounts for 3% of the WD mutations in our sample and produces an in-frame, 39-bp insertion in mRNA of patients homozygous, but not heterozygous, for the mutation. The most common WD mutation (His1069Glu) was represented in approximately 38% of all the WD chromosomes from the North American, Russian, and Swedish samples. In several population cohorts, this mutation deviated from Hardy-Weinberg equilibrium, with an overrepresentation of homozygotes. We did not find a significant correlation between His1069Glu homozygosity and several clinical indices, including age of onset, clinical manifestation, ceruloplasmin activity, hepatic copper levels, and the presence of Kayser-Fleischer rings. Finally, lymphoblast cell lines from individuals homozygous for His1069Glu and 4 other mutations all demonstrated significantly decreased copper-stimulated ATPase activity.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen J. P., Vilsen B. Structure-function relationships of cation translocation by Ca(2+)- and Na+, K(+)-ATPases studied by site-directed mutagenesis. FEBS Lett. 1995 Feb 13;359(2-3):101–106. doi: 10.1016/0014-5793(95)00019-6. [DOI] [PubMed] [Google Scholar]
- Bull P. C., Thomas G. R., Rommens J. M., Forbes J. R., Cox D. W. The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene. Nat Genet. 1993 Dec;5(4):327–337. doi: 10.1038/ng1293-327. [DOI] [PubMed] [Google Scholar]
- Chelly J., Tümer Z., Tønnesen T., Petterson A., Ishikawa-Brush Y., Tommerup N., Horn N., Monaco A. P. Isolation of a candidate gene for Menkes disease that encodes a potential heavy metal binding protein. Nat Genet. 1993 Jan;3(1):14–19. doi: 10.1038/ng0193-14. [DOI] [PubMed] [Google Scholar]
- Clarke D. M., Loo T. W., MacLennan D. H. Functional consequences of alterations to amino acids located in the nucleotide binding domain of the Ca2(+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1990 Dec 25;265(36):22223–22227. [PubMed] [Google Scholar]
- Das S., Levinson B., Vulpe C., Whitney S., Gitschier J., Packman S. Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am J Hum Genet. 1995 Mar;56(3):570–576. [PMC free article] [PubMed] [Google Scholar]
- Das S., Levinson B., Whitney S., Vulpe C., Packman S., Gitschier J. Diverse mutations in patients with Menkes disease often lead to exon skipping. Am J Hum Genet. 1994 Nov;55(5):883–889. [PMC free article] [PubMed] [Google Scholar]
- Don R. H., Cox P. T., Wainwright B. J., Baker K., Mattick J. S. 'Touchdown' PCR to circumvent spurious priming during gene amplification. Nucleic Acids Res. 1991 Jul 25;19(14):4008–4008. doi: 10.1093/nar/19.14.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figus A., Angius A., Loudianos G., Bertini C., Dessi V., Loi A., Deiana M., Lovicu M., Olla N., Sole G. Molecular pathology and haplotype analysis of Wilson disease in Mediterranean populations. Am J Hum Genet. 1995 Dec;57(6):1318–1324. [PMC free article] [PubMed] [Google Scholar]
- Freimer N. B., Reus V. I., Escamilla M. A., McInnes L. A., Spesny M., Leon P., Service S. K., Smith L. B., Silva S., Rojas E. Genetic mapping using haplotype, association and linkage methods suggests a locus for severe bipolar disorder (BPI) at 18q22-q23. Nat Genet. 1996 Apr;12(4):436–441. doi: 10.1038/ng0496-436. [DOI] [PubMed] [Google Scholar]
- Houwen R. H., Juyn J., Hoogenraad T. U., Ploos van Amstel J. K., Berger R. H714Q mutation in Wilson disease is associated with late, neurological presentation. J Med Genet. 1995 Jun;32(6):480–482. doi: 10.1136/jmg.32.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungmann J., Reins H. A., Lee J., Romeo A., Hassett R., Kosman D., Jentsch S. MAC1, a nuclear regulatory protein related to Cu-dependent transcription factors is involved in Cu/Fe utilization and stress resistance in yeast. EMBO J. 1993 Dec 15;12(13):5051–5056. doi: 10.1002/j.1460-2075.1993.tb06198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaler S. G., Gallo L. K., Proud V. K., Percy A. K., Mark Y., Segal N. A., Goldstein D. S., Holmes C. S., Gahl W. A. Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat Genet. 1994 Oct;8(2):195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- Kemppainen R., Palatsi R., Kallioinen M., Oikarinen A. A homozygous nonsense mutation and a combination of two mutations of the Wilson disease gene in patients with different lysyl oxidase activities in cultured fibroblasts. J Invest Dermatol. 1997 Jan;108(1):35–39. doi: 10.1111/1523-1747.ep12285622. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Loudianos G., Dessì V., Angius A., Lovicu M., Loi A., Deiana M., Akar N., Vajro P., Figus A., Cao A. Wilson disease mutations associated with uncommon haplotypes in Mediterranean patients. Hum Genet. 1996 Dec;98(6):640–642. doi: 10.1007/s004390050275. [DOI] [PubMed] [Google Scholar]
- Luza S. C., Speisky H. C. Liver copper storage and transport during development: implications for cytotoxicity. Am J Clin Nutr. 1996 May;63(5):812S–820S. doi: 10.1093/ajcn/63.5.812. [DOI] [PubMed] [Google Scholar]
- López de Castro J. A. Purification of human HLA-A and HLA-B class I histocompatibility antigens. Methods Enzymol. 1984;108:582–600. doi: 10.1016/s0076-6879(84)08119-2. [DOI] [PubMed] [Google Scholar]
- Mercer J. F., Livingston J., Hall B., Paynter J. A., Begy C., Chandrasekharappa S., Lockhart P., Grimes A., Bhave M., Siemieniak D. Isolation of a partial candidate gene for Menkes disease by positional cloning. Nat Genet. 1993 Jan;3(1):20–25. doi: 10.1038/ng0193-20. [DOI] [PubMed] [Google Scholar]
- Petrukhin K., Fischer S. G., Pirastu M., Tanzi R. E., Chernov I., Devoto M., Brzustowicz L. M., Cayanis E., Vitale E., Russo J. J. Mapping, cloning and genetic characterization of the region containing the Wilson disease gene. Nat Genet. 1993 Dec;5(4):338–343. doi: 10.1038/ng1293-338. [DOI] [PubMed] [Google Scholar]
- Petrukhin K., Gilliam T. C. Genetic disorders of copper metabolism. Curr Opin Pediatr. 1994 Dec;6(6):698–701. doi: 10.1097/00008480-199412000-00015. [DOI] [PubMed] [Google Scholar]
- Petrukhin K., Lutsenko S., Chernov I., Ross B. M., Kaplan J. H., Gilliam T. C. Characterization of the Wilson disease gene encoding a P-type copper transporting ATPase: genomic organization, alternative splicing, and structure/function predictions. Hum Mol Genet. 1994 Sep;3(9):1647–1656. doi: 10.1093/hmg/3.9.1647. [DOI] [PubMed] [Google Scholar]
- Shimizu N., Kawase C., Nakazono H., Hemmi H., Shimatake H., Aoki T. A novel RNA splicing mutation in Japanese patients with Wilson disease. Biochem Biophys Res Commun. 1995 Dec 5;217(1):16–20. doi: 10.1006/bbrc.1995.2739. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Petrukhin K., Chernov I., Pellequer J. L., Wasco W., Ross B., Romano D. M., Parano E., Pavone L., Brzustowicz L. M. The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet. 1993 Dec;5(4):344–350. doi: 10.1038/ng1293-344. [DOI] [PubMed] [Google Scholar]
- Vulpe C., Levinson B., Whitney S., Packman S., Gitschier J. Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet. 1993 Jan;3(1):7–13. doi: 10.1038/ng0193-7. [DOI] [PubMed] [Google Scholar]
- Waldenström E., Lagerkvist A., Dahlman T., Westermark K., Landegren U. Efficient detection of mutations in Wilson disease by manifold sequencing. Genomics. 1996 Nov 1;37(3):303–309. doi: 10.1006/geno.1996.0564. [DOI] [PubMed] [Google Scholar]
- Wu J., Forbes J. R., Chen H. S., Cox D. W. The LEC rat has a deletion in the copper transporting ATPase gene homologous to the Wilson disease gene. Nat Genet. 1994 Aug;7(4):541–545. doi: 10.1038/ng0894-541. [DOI] [PubMed] [Google Scholar]
- Yuan D. S., Stearman R., Dancis A., Dunn T., Beeler T., Klausner R. D. The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):2632–2636. doi: 10.1073/pnas.92.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Coyne M. Y., Will S. G., Levenson C. H., Kawasaki E. S. Single-base mutational analysis of cancer and genetic diseases using membrane bound modified oligonucleotides. Nucleic Acids Res. 1991 Jul 25;19(14):3929–3933. doi: 10.1093/nar/19.14.3929. [DOI] [PMC free article] [PubMed] [Google Scholar]