Abstract
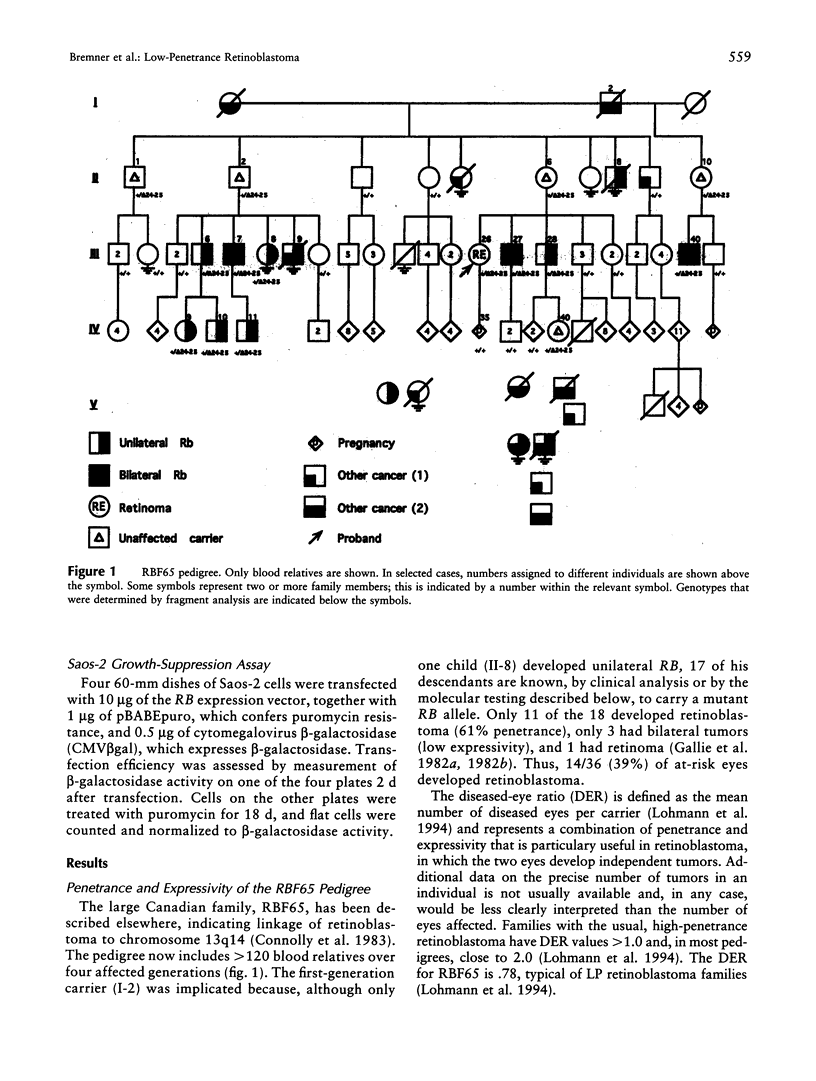
A deletion in the tumor-suppressor gene, RB, discovered by quantitative multiplex PCR, shows low penetrance (LP), since only 39% of eyes at risk in this family develop retinoblastoma. The 4-kb deletion spanning exons 24 and 25 (delta24-25) is the largest ever observed in an LP retinoblastoma family. Unlike the usual RB mutations, which cause retinoblastoma in 95% of at-risk eyes and yield no detectable protein, the delta24-25 allele transcribed a message splicing exon 23 to exon 26, resulting in a detectable protein (pRBdelta24-25) that lacks 58 amino acids from the C-terminal domain, proving that this domain is essential for suppression of retinoblastoma. Two functions were partially impaired by delta24-25-nuclear localization and repression of E2F-consistent with the idea that LP mutations generate "weak alleles" by reducing but not eliminating essential activities. However, delta24-25 ablated interaction of pRB with MDM2. Since a homozygous LP allele is considered nontumorigenic, the pRB/MDM2 interaction may be semi- or nonessential for suppressing retinoblastoma. Alternatively, some homozygous LP alleles may not cause tumorigenesis because an additional event is required (the "three-hit hypothesis"), or the resulting imbalance in pRB function may cause apoptosis (the "death allele hypothesis"). pRBdelta24-25 was also completely defective in suppressing growth of Saos-2 osteosarcoma cells. Targeting pRBdelta24-25 to the nucleus did not improve Saos-2 growth suppression, suggesting that C-terminal domain functions other than nuclear localization are essential for blocking proliferation in these cells. Since delta24-25 behaves like a null allele in these cells but like an LP allele in the retina, pRB may use different mechanisms to control growth in different cell types.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adnane J., Shao Z., Robbins P. D. The retinoblastoma susceptibility gene product represses transcription when directly bound to the promoter. J Biol Chem. 1995 Apr 14;270(15):8837–8843. doi: 10.1074/jbc.270.15.8837. [DOI] [PubMed] [Google Scholar]
- Aguzzi A., Brandner S., Isenmann S., Steinbach J. P., Sure U. Transgenic and gene disruption techniques in the study of neurocarcinogenesis. Glia. 1995 Nov;15(3):348–364. doi: 10.1002/glia.440150314. [DOI] [PubMed] [Google Scholar]
- An B., Jin J. R., Lin P., Dou Q. P. Failure to activate interleukin 1beta-converting enzyme-like proteases and to cleave retinoblastoma protein in drug-resistant cells. FEBS Lett. 1996 Dec 9;399(1-2):158–162. doi: 10.1016/s0014-5793(96)01311-7. [DOI] [PubMed] [Google Scholar]
- Barnes W. M. PCR amplification of up to 35-kb DNA with high fidelity and high yield from lambda bacteriophage templates. Proc Natl Acad Sci U S A. 1994 Mar 15;91(6):2216–2220. doi: 10.1073/pnas.91.6.2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista D., Emanuel J. R., Granville C., Howard R., Costa J. Identification of mutations in the Ki-ras gene in human retinoblastoma. Invest Ophthalmol Vis Sci. 1996 Oct;37(11):2313–2317. [PubMed] [Google Scholar]
- Benedict W. F., Murphree A. L., Banerjee A., Spina C. A., Sparkes M. C., Sparkes R. S. Patient with 13 chromosome deletion: evidence that the retinoblastoma gene is a recessive cancer gene. Science. 1983 Feb 25;219(4587):973–975. doi: 10.1126/science.6336308. [DOI] [PubMed] [Google Scholar]
- Bia B., Cowell J. K. Independent constitutional germline mutations occurring in the RB1 gene in cousins with bilateral retinoblastoma. Oncogene. 1995 Sep 7;11(5):977–979. [PubMed] [Google Scholar]
- Blanquet V., Turleau C., Gross M. S., Goossens M., Besmond C. Identification of germline mutations in the RB1 gene by denaturant gradient gel electrophoresis and polymerase chain reaction direct sequencing. Hum Mol Genet. 1993 Jul;2(7):975–979. doi: 10.1093/hmg/2.7.975. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Shew J. Y., Chen P. L., Scully P., Lee W. H. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science. 1990 Feb 9;247(4943):712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- Bremner R., Cohen B. L., Sopta M., Hamel P. A., Ingles C. J., Gallie B. L., Phillips R. A. Direct transcriptional repression by pRB and its reversal by specific cyclins. Mol Cell Biol. 1995 Jun;15(6):3256–3265. doi: 10.1128/mcb.15.6.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Canning S., Dryja T. P. Short, direct repeats at the breakpoints of deletions of the retinoblastoma gene. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5044–5048. doi: 10.1073/pnas.86.13.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavenee W. K., Dryja T. P., Phillips R. A., Benedict W. F., Godbout R., Gallie B. L., Murphree A. L., Strong L. C., White R. L. Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. 1983 Oct 27-Nov 2Nature. 305(5937):779–784. doi: 10.1038/305779a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. S., Chamberlain J. R., Fenwick R. G., Ward P. A., Caskey C. T., Dimnik L. S., Bech-Hansen N. T., Hoar D. I., Richards S., Covone A. E. Diagnosis of Duchenne and Becker muscular dystrophies by polymerase chain reaction. A multicenter study. JAMA. 1992 May 20;267(19):2609–2615. doi: 10.1001/jama.1992.03480190051030. [DOI] [PubMed] [Google Scholar]
- Chen P. L., Scully P., Shew J. Y., Wang J. Y., Lee W. H. Phosphorylation of the retinoblastoma gene product is modulated during the cell cycle and cellular differentiation. Cell. 1989 Sep 22;58(6):1193–1198. doi: 10.1016/0092-8674(89)90517-5. [DOI] [PubMed] [Google Scholar]
- Chen W. D., Otterson G. A., Lipkowitz S., Khleif S. N., Coxon A. B., Kaye F. J. Apoptosis is associated with cleavage of a 5 kDa fragment from RB which mimics dephosphorylation and modulates E2F binding. Oncogene. 1997 Mar 13;14(10):1243–1248. doi: 10.1038/sj.onc.1201096. [DOI] [PubMed] [Google Scholar]
- Cheng S., Fockler C., Barnes W. M., Higuchi R. Effective amplification of long targets from cloned inserts and human genomic DNA. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5695–5699. doi: 10.1073/pnas.91.12.5695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly M. J., Payne R. H., Johnson G., Gallie B. L., Allderdice P. W., Marshall W. H., Lawton R. D. Familial, EsD-linked, retinoblastoma with reduced penetrance and variable expressivity. Hum Genet. 1983;65(2):122–124. doi: 10.1007/BF00286647. [DOI] [PubMed] [Google Scholar]
- Cordon-Cardo C., Latres E., Drobnjak M., Oliva M. R., Pollack D., Woodruff J. M., Marechal V., Chen J., Brennan M. F., Levine A. J. Molecular abnormalities of mdm2 and p53 genes in adult soft tissue sarcomas. Cancer Res. 1994 Feb 1;54(3):794–799. [PubMed] [Google Scholar]
- Cowell J. K., Bia B., Akoulitchev A. A novel mutation in the promotor region in a family with a mild form of retinoblastoma indicates the location of a new regulatory domain for the RB1 gene. Oncogene. 1996 Jan 18;12(2):431–436. [PubMed] [Google Scholar]
- Cowell J. K., Smith T., Bia B. Frequent constitutional C to T mutations in CGA-arginine codons in the RB1 gene produce premature stop codons in patients with bilateral (hereditary) retinoblastoma. Eur J Hum Genet. 1994;2(4):281–290. doi: 10.1159/000472372. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Figge J., Shew J. Y., Huang C. M., Lee W. H., Marsilio E., Paucha E., Livingston D. M. SV40 large tumor antigen forms a specific complex with the product of the retinoblastoma susceptibility gene. Cell. 1988 Jul 15;54(2):275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- DeCaprio J. A., Ludlow J. W., Lynch D., Furukawa Y., Griffin J., Piwnica-Worms H., Huang C. M., Livingston D. M. The product of the retinoblastoma susceptibility gene has properties of a cell cycle regulatory element. Cell. 1989 Sep 22;58(6):1085–1095. doi: 10.1016/0092-8674(89)90507-2. [DOI] [PubMed] [Google Scholar]
- DeGregori J., Kowalik T., Nevins J. R. Cellular targets for activation by the E2F1 transcription factor include DNA synthesis- and G1/S-regulatory genes. Mol Cell Biol. 1995 Aug;15(8):4215–4224. doi: 10.1128/mcb.15.8.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeo-Jones D., Huang P. S., Jones R. E., Haskell K. M., Vuocolo G. A., Hanobik M. G., Huber H. E., Oliff A. Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature. 1991 Jul 18;352(6332):251–254. doi: 10.1038/352251a0. [DOI] [PubMed] [Google Scholar]
- Dowdy S. F., Hinds P. W., Louie K., Reed S. I., Arnold A., Weinberg R. A. Physical interaction of the retinoblastoma protein with human D cyclins. Cell. 1993 May 7;73(3):499–511. doi: 10.1016/0092-8674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Draper G. J., Sanders B. M., Kingston J. E. Second primary neoplasms in patients with retinoblastoma. Br J Cancer. 1986 May;53(5):661–671. doi: 10.1038/bjc.1986.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Rapaport J., McGee T. L., Nork T. M., Schwartz T. L. Molecular etiology of low-penetrance retinoblastoma in two pedigrees. Am J Hum Genet. 1993 Jun;52(6):1122–1128. [PMC free article] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duronio R. J., O'Farrell P. H. Developmental control of the G1 to S transition in Drosophila: cyclin Eis a limiting downstream target of E2F. Genes Dev. 1995 Jun 15;9(12):1456–1468. doi: 10.1101/gad.9.12.1456. [DOI] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Eng C., Li F. P., Abramson D. H., Ellsworth R. M., Wong F. L., Goldman M. B., Seddon J., Tarbell N., Boice J. D., Jr Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst. 1993 Jul 21;85(14):1121–1128. doi: 10.1093/jnci/85.14.1121. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Sluss H. K., Sherr C. J., Matsushime H., Kato J., Livingston D. M. Functional interactions of the retinoblastoma protein with mammalian D-type cyclins. Cell. 1993 May 7;73(3):487–497. doi: 10.1016/0092-8674(93)90136-e. [DOI] [PubMed] [Google Scholar]
- Ewen M. E., Xing Y. G., Lawrence J. B., Livingston D. M. Molecular cloning, chromosomal mapping, and expression of the cDNA for p107, a retinoblastoma gene product-related protein. Cell. 1991 Sep 20;66(6):1155–1164. doi: 10.1016/0092-8674(91)90038-z. [DOI] [PubMed] [Google Scholar]
- Finlay C. A. The mdm-2 oncogene can overcome wild-type p53 suppression of transformed cell growth. Mol Cell Biol. 1993 Jan;13(1):301–306. doi: 10.1128/mcb.13.1.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemington E. K., Speck S. H., Kaelin W. G., Jr E2F-1-mediated transactivation is inhibited by complex formation with the retinoblastoma susceptibility gene product. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):6914–6918. doi: 10.1073/pnas.90.15.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie B. L., Ellsworth R. M., Abramson D. H., Phillips R. A. Retinoma: spontaneous regression of retinoblastoma or benign manifestation of the mutation? Br J Cancer. 1982 Apr;45(4):513–521. doi: 10.1038/bjc.1982.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallie B. L., Phillips R. A., Ellsworth R. M., Abramson D. H. Significance of retinoma and phthisis bulbi for retinoblastoma. Ophthalmology. 1982 Dec;89(12):1393–1399. doi: 10.1016/s0161-6420(82)34622-9. [DOI] [PubMed] [Google Scholar]
- Goddard A. D., Balakier H., Canton M., Dunn J., Squire J., Reyes E., Becker A., Phillips R. A., Gallie B. L. Infrequent genomic rearrangement and normal expression of the putative RB1 gene in retinoblastoma tumors. Mol Cell Biol. 1988 May;8(5):2082–2088. doi: 10.1128/mcb.8.5.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich D. W., Wang N. P., Qian Y. W., Lee E. Y., Lee W. H. The retinoblastoma gene product regulates progression through the G1 phase of the cell cycle. Cell. 1991 Oct 18;67(2):293–302. doi: 10.1016/0092-8674(91)90181-w. [DOI] [PubMed] [Google Scholar]
- Hagemeier C., Bannister A. J., Cook A., Kouzarides T. The activation domain of transcription factor PU.1 binds the retinoblastoma (RB) protein and the transcription factor TFIID in vitro: RB shows sequence similarity to TFIID and TFIIB. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1580–1584. doi: 10.1073/pnas.90.4.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemeier C., Cook A., Kouzarides T. The retinoblastoma protein binds E2F residues required for activation in vivo and TBP binding in vitro. Nucleic Acids Res. 1993 Nov 11;21(22):4998–5004. doi: 10.1093/nar/21.22.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel P. A., Cohen B. L., Sorce L. M., Gallie B. L., Phillips R. A. Hyperphosphorylation of the retinoblastoma gene product is determined by domains outside the simian virus 40 large-T-antigen-binding regions. Mol Cell Biol. 1990 Dec;10(12):6586–6595. doi: 10.1128/mcb.10.12.6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel P. A., Gill R. M., Phillips R. A., Gallie B. L. Transcriptional repression of the E2-containing promoters EIIaE, c-myc, and RB1 by the product of the RB1 gene. Mol Cell Biol. 1992 Aug;12(8):3431–3438. doi: 10.1128/mcb.12.8.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon G. J., Demetrick D., Beach D. Isolation of the Rb-related p130 through its interaction with CDK2 and cyclins. Genes Dev. 1993 Dec;7(12A):2378–2391. doi: 10.1101/gad.7.12a.2378. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Morgan R., Sandberg A. A., Cavenee W. K. Structural alterations at the putative retinoblastoma locus in some human leukemias and preleukemia. Cancer Genet Cytogenet. 1990 Oct 1;49(1):15–23. doi: 10.1016/0165-4608(90)90159-8. [DOI] [PubMed] [Google Scholar]
- Harbour J. W., Lai S. L., Whang-Peng J., Gazdar A. F., Minna J. D., Kaye F. J. Abnormalities in structure and expression of the human retinoblastoma gene in SCLC. Science. 1988 Jul 15;241(4863):353–357. doi: 10.1126/science.2838909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison D. J., Hooper M. L., Armstrong J. F., Clarke A. R. Effects of heterozygosity for the Rb-1t19neo allele in the mouse. Oncogene. 1995 Apr 20;10(8):1615–1620. [PubMed] [Google Scholar]
- Hashimoto T., Takahashi R., Yandell D. W., Xu H. J., Hu S. X., Gunnell S., Benedict W. F. Characterization of intragenic deletions in two sporadic germinal mutation cases of retinoblastoma resulting in abnormal gene expression. Oncogene. 1991 Mar;6(3):463–469. [PubMed] [Google Scholar]
- Helin K., Harlow E., Fattaey A. Inhibition of E2F-1 transactivation by direct binding of the retinoblastoma protein. Mol Cell Biol. 1993 Oct;13(10):6501–6508. doi: 10.1128/mcb.13.10.6501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann J. Delayed mutation as a cause of retinoblastoma: application to genetic counseling. Birth Defects Orig Artic Ser. 1976;12(1):79–90. [PubMed] [Google Scholar]
- Hiebert S. W., Chellappan S. P., Horowitz J. M., Nevins J. R. The interaction of RB with E2F coincides with an inhibition of the transcriptional activity of E2F. Genes Dev. 1992 Feb;6(2):177–185. doi: 10.1101/gad.6.2.177. [DOI] [PubMed] [Google Scholar]
- Hiebert S. W. Regions of the retinoblastoma gene product required for its interaction with the E2F transcription factor are necessary for E2 promoter repression and pRb-mediated growth suppression. Mol Cell Biol. 1993 Jun;13(6):3384–3391. doi: 10.1128/mcb.13.6.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P. W., Mittnacht S., Dulic V., Arnold A., Reed S. I., Weinberg R. A. Regulation of retinoblastoma protein functions by ectopic expression of human cyclins. Cell. 1992 Sep 18;70(6):993–1006. doi: 10.1016/0092-8674(92)90249-c. [DOI] [PubMed] [Google Scholar]
- Hogg A., Bia B., Onadim Z., Cowell J. K. Molecular mechanisms of oncogenic mutations in tumors from patients with bilateral and unilateral retinoblastoma. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7351–7355. doi: 10.1073/pnas.90.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Park S. H., Bogenmann E., Cheng J. C., Yandell D. W., Kaye F. J., Minna J. D., Dryja T. P., Weinberg R. A. Frequent inactivation of the retinoblastoma anti-oncogene is restricted to a subset of human tumor cells. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2775–2779. doi: 10.1073/pnas.87.7.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Hu N., Gutsmann A., Herbert D. C., Bradley A., Lee W. H., Lee E. Y. Heterozygous Rb-1 delta 20/+mice are predisposed to tumors of the pituitary gland with a nearly complete penetrance. Oncogene. 1994 Apr;9(4):1021–1027. [PubMed] [Google Scholar]
- Hu Q. J., Dyson N., Harlow E. The regions of the retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J. 1990 Apr;9(4):1147–1155. doi: 10.1002/j.1460-2075.1990.tb08221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S., Lee W. H., Lee E. Y. A cellular protein that competes with SV40 T antigen for binding to the retinoblastoma gene product. Nature. 1991 Mar 14;350(6314):160–162. doi: 10.1038/350160a0. [DOI] [PubMed] [Google Scholar]
- Ioannou P., Christopoulos G., Panayides K., Kleanthous M., Middleton L. Detection of Duchenne and Becker muscular dystrophy carriers by quantitative multiplex polymerase chain reaction analysis. Neurology. 1992 Sep;42(9):1783–1790. doi: 10.1212/wnl.42.9.1783. [DOI] [PubMed] [Google Scholar]
- Jacks T., Fazeli A., Schmitt E. M., Bronson R. T., Goodell M. A., Weinberg R. A. Effects of an Rb mutation in the mouse. Nature. 1992 Sep 24;359(6393):295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- Jänicke R. U., Walker P. A., Lin X. Y., Porter A. G. Specific cleavage of the retinoblastoma protein by an ICE-like protease in apoptosis. EMBO J. 1996 Dec 16;15(24):6969–6978. [PMC free article] [PubMed] [Google Scholar]
- Kato G., Wakabayashi K. Effect of polylysine-bound laminin on human retinoblastoma cell lines. In Vitro Cell Dev Biol. 1988 Apr;24(4):274–280. doi: 10.1007/BF02628827. [DOI] [PubMed] [Google Scholar]
- Kato M. V., Ishizaki K., Toguchida J., Kaneko A., Takayama J., Tanooka H., Kato T., Shimizu T., Sasaki M. S. Mutations in the retinoblastoma gene and their expression in somatic and tumor cells of patients with hereditary retinoblastoma. Hum Mutat. 1994;3(1):44–51. doi: 10.1002/humu.1380030108. [DOI] [PubMed] [Google Scholar]
- Kaye F. J., Kratzke R. A., Gerster J. L., Horowitz J. M. A single amino acid substitution results in a retinoblastoma protein defective in phosphorylation and oncoprotein binding. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6922–6926. doi: 10.1073/pnas.87.17.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kratzke R. A., Otterson G. A., Hogg A., Coxon A. B., Geradts J., Cowell J. K., Kaye F. J. Partial inactivation of the RB product in a family with incomplete penetrance of familial retinoblastoma and benign retinal tumors. Oncogene. 1994 May;9(5):1321–1326. [PubMed] [Google Scholar]
- Kratzke R. A., Otterson G. A., Lin A. Y., Shimizu E., Alexandrova N., Zajac-Kaye M., Horowitz J. M., Kaye F. J. Functional analysis at the Cys706 residue of the retinoblastoma protein. J Biol Chem. 1992 Dec 25;267(36):25998–26003. [PubMed] [Google Scholar]
- La Thangue N. B. DRTF1/E2F: an expanding family of heterodimeric transcription factors implicated in cell-cycle control. Trends Biochem Sci. 1994 Mar;19(3):108–114. doi: 10.1016/0968-0004(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Ladanyi M., Cha C., Lewis R., Jhanwar S. C., Huvos A. G., Healey J. H. MDM2 gene amplification in metastatic osteosarcoma. Cancer Res. 1993 Jan 1;53(1):16–18. [PubMed] [Google Scholar]
- Leach F. S., Tokino T., Meltzer P., Burrell M., Oliner J. D., Smith S., Hill D. E., Sidransky D., Kinzler K. W., Vogelstein B. p53 Mutation and MDM2 amplification in human soft tissue sarcomas. Cancer Res. 1993 May 15;53(10 Suppl):2231–2234. [PubMed] [Google Scholar]
- Lee E. Y., Chang C. Y., Hu N., Wang Y. C., Lai C. C., Herrup K., Lee W. H., Bradley A. Mice deficient for Rb are nonviable and show defects in neurogenesis and haematopoiesis. Nature. 1992 Sep 24;359(6393):288–294. doi: 10.1038/359288a0. [DOI] [PubMed] [Google Scholar]
- Li Y., Graham C., Lacy S., Duncan A. M., Whyte P. The adenovirus E1A-associated 130-kD protein is encoded by a member of the retinoblastoma gene family and physically interacts with cyclins A and E. Genes Dev. 1993 Dec;7(12A):2366–2377. doi: 10.1101/gad.7.12a.2366. [DOI] [PubMed] [Google Scholar]
- Lohmann D. R., Brandt B., Höpping W., Passarge E., Horsthemke B. Distinct RB1 gene mutations with low penetrance in hereditary retinoblastoma. Hum Genet. 1994 Oct;94(4):349–354. doi: 10.1007/BF00201591. [DOI] [PubMed] [Google Scholar]
- Lohmann D., Horsthemke B., Gillessen-Kaesbach G., Stefani F. H., Höfler H. Detection of small RB1 gene deletions in retinoblastoma by multiplex PCR and high-resolution gel electrophoresis. Hum Genet. 1992 Apr;89(1):49–53. doi: 10.1007/BF00207041. [DOI] [PubMed] [Google Scholar]
- Lundgren K., Montes de Oca Luna R., McNeill Y. B., Emerick E. P., Spencer B., Barfield C. R., Lozano G., Rosenberg M. P., Finlay C. A. Targeted expression of MDM2 uncouples S phase from mitosis and inhibits mammary gland development independent of p53. Genes Dev. 1997 Mar 15;11(6):714–725. doi: 10.1101/gad.11.6.714. [DOI] [PubMed] [Google Scholar]
- MACKLIN M. T. A study of retinoblastoma in Ohio. Am J Hum Genet. 1960 Mar;12:1–43. [PMC free article] [PubMed] [Google Scholar]
- Maandag E. C., van der Valk M., Vlaar M., Feltkamp C., O'Brien J., van Roon M., van der Lugt N., Berns A., te Riele H. Developmental rescue of an embryonic-lethal mutation in the retinoblastoma gene in chimeric mice. EMBO J. 1994 Sep 15;13(18):4260–4268. doi: 10.1002/j.1460-2075.1994.tb06746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunaga E. Hereditary retinoblastoma: delayed mutation or host resistance? Am J Hum Genet. 1978 Jul;30(4):406–424. [PMC free article] [PubMed] [Google Scholar]
- Mayol X., Graña X., Baldi A., Sang N., Hu Q., Giordano A. Cloning of a new member of the retinoblastoma gene family (pRb2) which binds to the E1A transforming domain. Oncogene. 1993 Sep;8(9):2561–2566. [PubMed] [Google Scholar]
- McFall R. C., Sery T. W., Makadon M. Characterization of a new continuous cell line derived from a human retinoblastoma. Cancer Res. 1977 Apr;37(4):1003–1010. [PubMed] [Google Scholar]
- Munier F. L., Wang M. X., Spence M. A., Thonney F., Balmer A., Pescia G., Donoso L. A., Murphree A. L. Pseudo low penetrance in retinoblastoma. Fortuitous familial aggregation of sporadic cases caused by independently derived mutations in two large pedigrees. Arch Ophthalmol. 1993 Nov;111(11):1507–1511. doi: 10.1001/archopht.1993.01090110073028. [DOI] [PubMed] [Google Scholar]
- Munier F., Spence M. A., Pescia G., Balmer A., Gailloud C., Thonney F., van Melle G., Rutz H. P. Paternal selection favoring mutant alleles of the retinoblastoma susceptibility gene. Hum Genet. 1992 Jul;89(5):508–512. doi: 10.1007/BF00219175. [DOI] [PubMed] [Google Scholar]
- Noorani H. Z., Khan H. N., Gallie B. L., Detsky A. S. Cost comparison of molecular versus conventional screening of relatives at risk for retinoblastoma. Am J Hum Genet. 1996 Aug;59(2):301–307. [PMC free article] [PubMed] [Google Scholar]
- Ohtani K., DeGregori J., Nevins J. R. Regulation of the cyclin E gene by transcription factor E2F1. Proc Natl Acad Sci U S A. 1995 Dec 19;92(26):12146–12150. doi: 10.1073/pnas.92.26.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliner J. D., Kinzler K. W., Meltzer P. S., George D. L., Vogelstein B. Amplification of a gene encoding a p53-associated protein in human sarcomas. Nature. 1992 Jul 2;358(6381):80–83. doi: 10.1038/358080a0. [DOI] [PubMed] [Google Scholar]
- Oliner J. D., Pietenpol J. A., Thiagalingam S., Gyuris J., Kinzler K. W., Vogelstein B. Oncoprotein MDM2 conceals the activation domain of tumour suppressor p53. Nature. 1993 Apr 29;362(6423):857–860. doi: 10.1038/362857a0. [DOI] [PubMed] [Google Scholar]
- Onadim Z. O., Mitchell C. D., Rutland P. C., Buckle B. G., Jay M., Hungerford J. L., Harper K., Cowell J. K. Application of intragenic DNA probes in prenatal screening for retinoblastoma gene carriers in the United Kingdom. Arch Dis Child. 1990 Jul;65(7 Spec No):651–656. doi: 10.1136/adc.65.7_spec_no.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onadim Z., Hogg A., Baird P. N., Cowell J. K. Oncogenic point mutations in exon 20 of the RB1 gene in families showing incomplete penetrance and mild expression of the retinoblastoma phenotype. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):6177–6181. doi: 10.1073/pnas.89.13.6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onadim Z., Hykin P. G., Hungerford J. L., Cowell J. K. Genetic counselling in retinoblastoma: importance of ocular fundus examination of first degree relatives and linkage analysis. Br J Ophthalmol. 1991 Mar;75(3):147–150. doi: 10.1136/bjo.75.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y., Luckey C., Horton L., Esser M., Templeton D. J. Biological function of the retinoblastoma protein requires distinct domains for hyperphosphorylation and transcription factor binding. Mol Cell Biol. 1992 Dec;12(12):5363–5372. doi: 10.1128/mcb.12.12.5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin X. Q., Chittenden T., Livingston D. M., Kaelin W. G., Jr Identification of a growth suppression domain within the retinoblastoma gene product. Genes Dev. 1992 Jun;6(6):953–964. doi: 10.1101/gad.6.6.953. [DOI] [PubMed] [Google Scholar]
- Reifenberger G., Liu L., Ichimura K., Schmidt E. E., Collins V. P. Amplification and overexpression of the MDM2 gene in a subset of human malignant gliomas without p53 mutations. Cancer Res. 1993 Jun 15;53(12):2736–2739. [PubMed] [Google Scholar]
- Sakai T., Ohtani N., McGee T. L., Robbins P. D., Dryja T. P. Oncogenic germ-line mutations in Sp1 and ATF sites in the human retinoblastoma gene. Nature. 1991 Sep 5;353(6339):83–86. doi: 10.1038/353083a0. [DOI] [PubMed] [Google Scholar]
- Schneider J. W., Gu W., Zhu L., Mahdavi V., Nadal-Ginard B. Reversal of terminal differentiation mediated by p107 in Rb-/- muscle cells. Science. 1994 Jun 3;264(5164):1467–1471. doi: 10.1126/science.8197461. [DOI] [PubMed] [Google Scholar]
- Schwartz L. S., Tarleton J., Popovich B., Seltzer W. K., Hoffman E. P. Fluorescent multiplex linkage analysis and carrier detection for Duchenne/Becker muscular dystrophy. Am J Hum Genet. 1992 Oct;51(4):721–729. [PMC free article] [PubMed] [Google Scholar]
- Sellers W. R., Rodgers J. W., Kaelin W. G., Jr A potent transrepression domain in the retinoblastoma protein induces a cell cycle arrest when bound to E2F sites. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11544–11548. doi: 10.1073/pnas.92.25.11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew J. Y., Lin B. T., Chen P. L., Tseng B. Y., Yang-Feng T. L., Lee W. H. C-terminal truncation of the retinoblastoma gene product leads to functional inactivation. Proc Natl Acad Sci U S A. 1990 Jan;87(1):6–10. doi: 10.1073/pnas.87.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire J., Phillips R. A., Boyce S., Godbout R., Rogers B., Gallie B. L. Isochromosome 6p, a unique chromosomal abnormality in retinoblastoma: verification by standard staining techniques, new densitometric methods, and somatic cell hybridization. Hum Genet. 1984;66(1):46–53. doi: 10.1007/BF00275185. [DOI] [PubMed] [Google Scholar]
- Stirdivant S. M., Ahern J. D., Oliff A., Heimbrook D. C. Retinoblastoma protein binding properties are dependent on 4 cysteine residues in the protein binding pocket. J Biol Chem. 1992 Jul 25;267(21):14846–14851. [PubMed] [Google Scholar]
- Strong L. C., Riccardi V. M., Ferrell R. E., Sparkes R. S. Familial retinoblastoma and chromosome 13 deletion transmitted via an insertional translocation. Science. 1981 Sep 25;213(4515):1501–1503. doi: 10.1126/science.7280668. [DOI] [PubMed] [Google Scholar]
- Tan X., Martin S. J., Green D. R., Wang J. Y. Degradation of retinoblastoma protein in tumor necrosis factor- and CD95-induced cell death. J Biol Chem. 1997 Apr 11;272(15):9613–9616. doi: 10.1074/jbc.272.15.9613. [DOI] [PubMed] [Google Scholar]
- Templeton D. J., Park S. H., Lanier L., Weinberg R. A. Nonfunctional mutants of the retinoblastoma protein are characterized by defects in phosphorylation, viral oncoprotein association, and nuclear tethering. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3033–3037. doi: 10.1073/pnas.88.8.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadayama B., Toguchida J., Shimizu T., Ishizaki K., Sasaki M. S., Kotoura Y., Yamamuro T. Mutation spectrum of the retinoblastoma gene in osteosarcomas. Cancer Res. 1994 Jun 1;54(11):3042–3048. [PubMed] [Google Scholar]
- Wang C. Y., Petryniak B., Thompson C. B., Kaelin W. G., Leiden J. M. Regulation of the Ets-related transcription factor Elf-1 by binding to the retinoblastoma protein. Science. 1993 May 28;260(5112):1330–1335. doi: 10.1126/science.8493578. [DOI] [PubMed] [Google Scholar]
- Welch P. J., Wang J. Y. A C-terminal protein-binding domain in the retinoblastoma protein regulates nuclear c-Abl tyrosine kinase in the cell cycle. Cell. 1993 Nov 19;75(4):779–790. doi: 10.1016/0092-8674(93)90497-e. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]
- Williams B. O., Remington L., Albert D. M., Mukai S., Bronson R. T., Jacks T. Cooperative tumorigenic effects of germline mutations in Rb and p53. Nat Genet. 1994 Aug;7(4):480–484. doi: 10.1038/ng0894-480. [DOI] [PubMed] [Google Scholar]
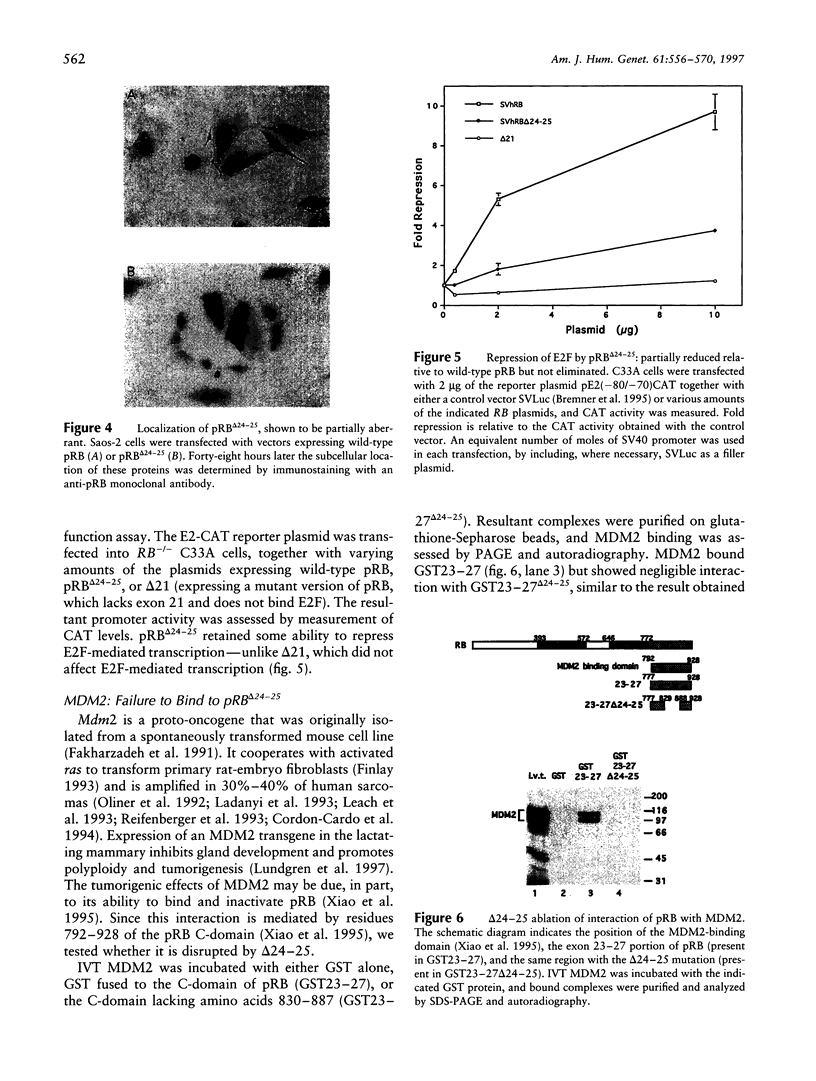
- Xiao Z. X., Chen J., Levine A. J., Modjtahedi N., Xing J., Sellers W. R., Livingston D. M. Interaction between the retinoblastoma protein and the oncoprotein MDM2. Nature. 1995 Jun 22;375(6533):694–698. doi: 10.1038/375694a0. [DOI] [PubMed] [Google Scholar]
- Yandell D. W., Campbell T. A., Dayton S. H., Petersen R., Walton D., Little J. B., McConkie-Rosell A., Buckley E. G., Dryja T. P. Oncogenic point mutations in the human retinoblastoma gene: their application to genetic counseling. N Engl J Med. 1989 Dec 21;321(25):1689–1695. doi: 10.1056/NEJM198912213212501. [DOI] [PubMed] [Google Scholar]
- Yokota J., Akiyama T., Fung Y. K., Benedict W. F., Namba Y., Hanaoka M., Wada M., Terasaki T., Shimosato Y., Sugimura T. Altered expression of the retinoblastoma (RB) gene in small-cell carcinoma of the lung. Oncogene. 1988 Oct;3(4):471–475. [PubMed] [Google Scholar]
- Zacksenhaus E., Bremner R., Phillips R. A., Gallie B. L. A bipartite nuclear localization signal in the retinoblastoma gene product and its importance for biological activity. Mol Cell Biol. 1993 Aug;13(8):4588–4599. doi: 10.1128/mcb.13.8.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacksenhaus E., Jiang Z., Phillips R. A., Gallie B. L. Dual mechanisms of repression of E2F1 activity by the retinoblastoma gene product. EMBO J. 1996 Nov 1;15(21):5917–5927. [PMC free article] [PubMed] [Google Scholar]
- Zhu L., van den Heuvel S., Helin K., Fattaey A., Ewen M., Livingston D., Dyson N., Harlow E. Inhibition of cell proliferation by p107, a relative of the retinoblastoma protein. Genes Dev. 1993 Jul;7(7A):1111–1125. doi: 10.1101/gad.7.7a.1111. [DOI] [PubMed] [Google Scholar]
- Zhu X. P., Dunn J. M., Phillips R. A., Goddard A. D., Paton K. E., Becker A., Gallie B. L. Preferential germline mutation of the paternal allele in retinoblastoma. Nature. 1989 Jul 27;340(6231):312–313. doi: 10.1038/340312a0. [DOI] [PubMed] [Google Scholar]
- de Oca Luna R. M., Tabor A. D., Eberspaecher H., Hulboy D. L., Worth L. L., Colman M. S., Finlay C. A., Lozano G. The organization and expression of the mdm2 gene. Genomics. 1996 May 1;33(3):352–357. doi: 10.1006/geno.1996.0210. [DOI] [PubMed] [Google Scholar]