Abstract
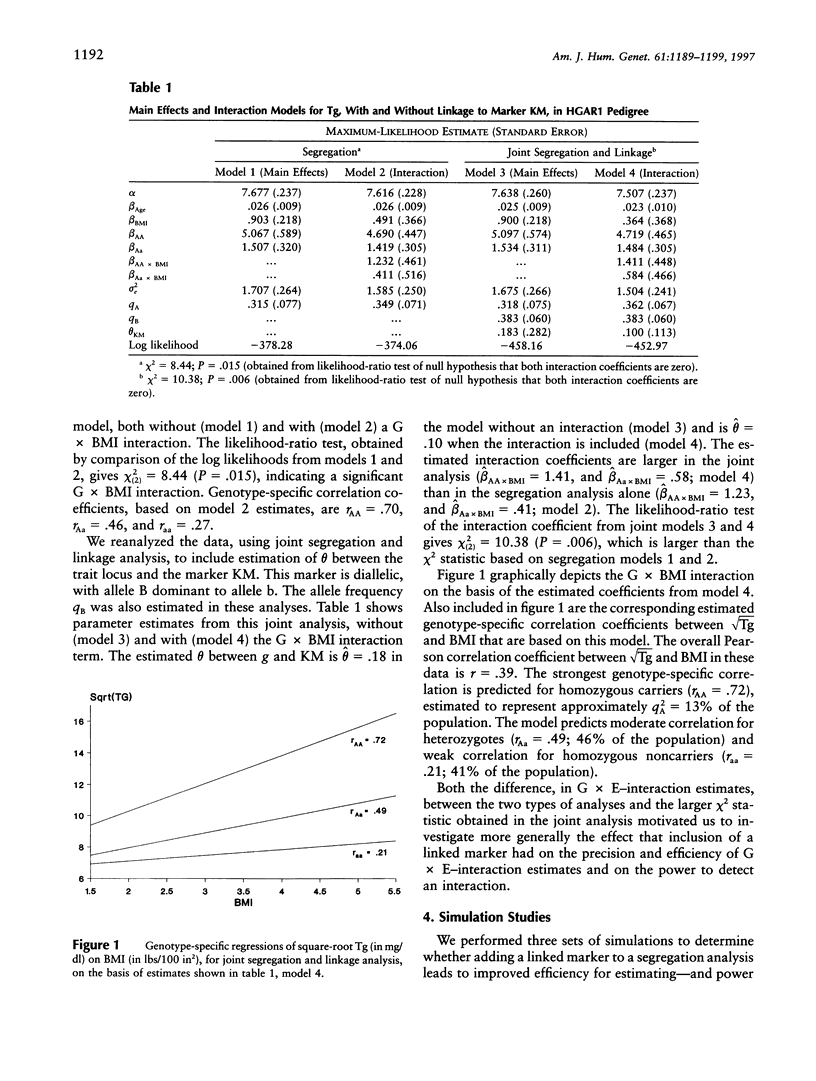
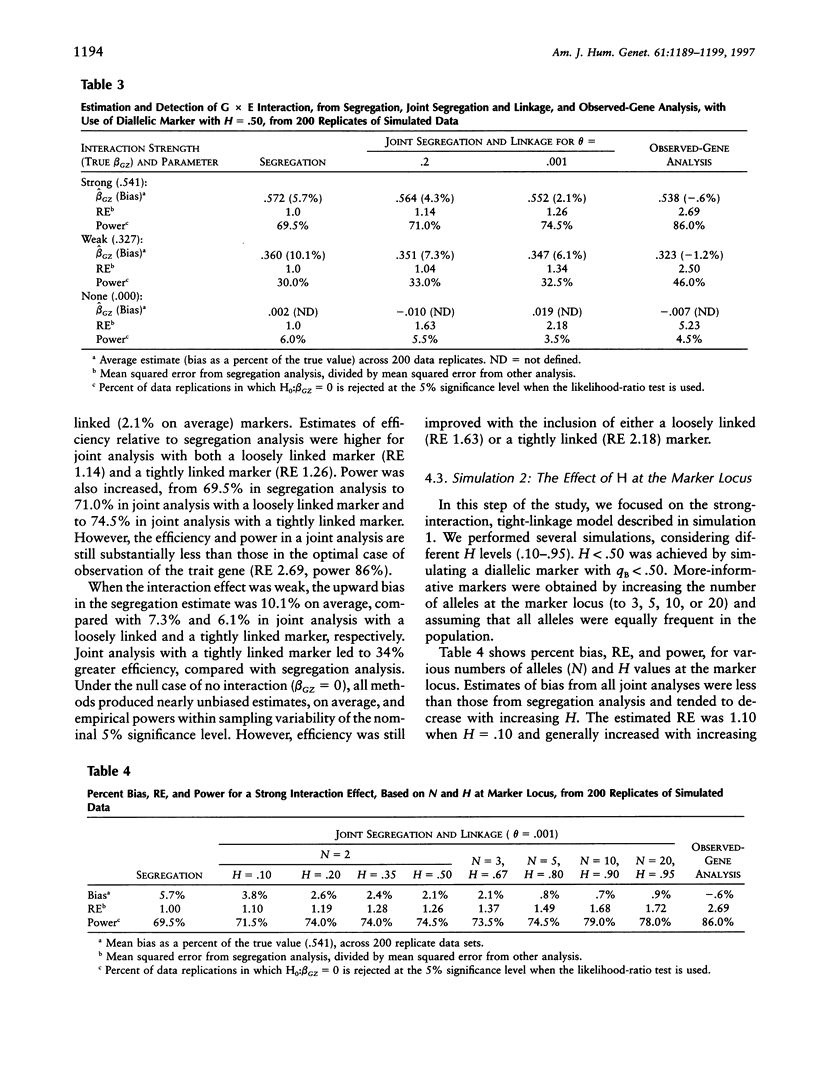
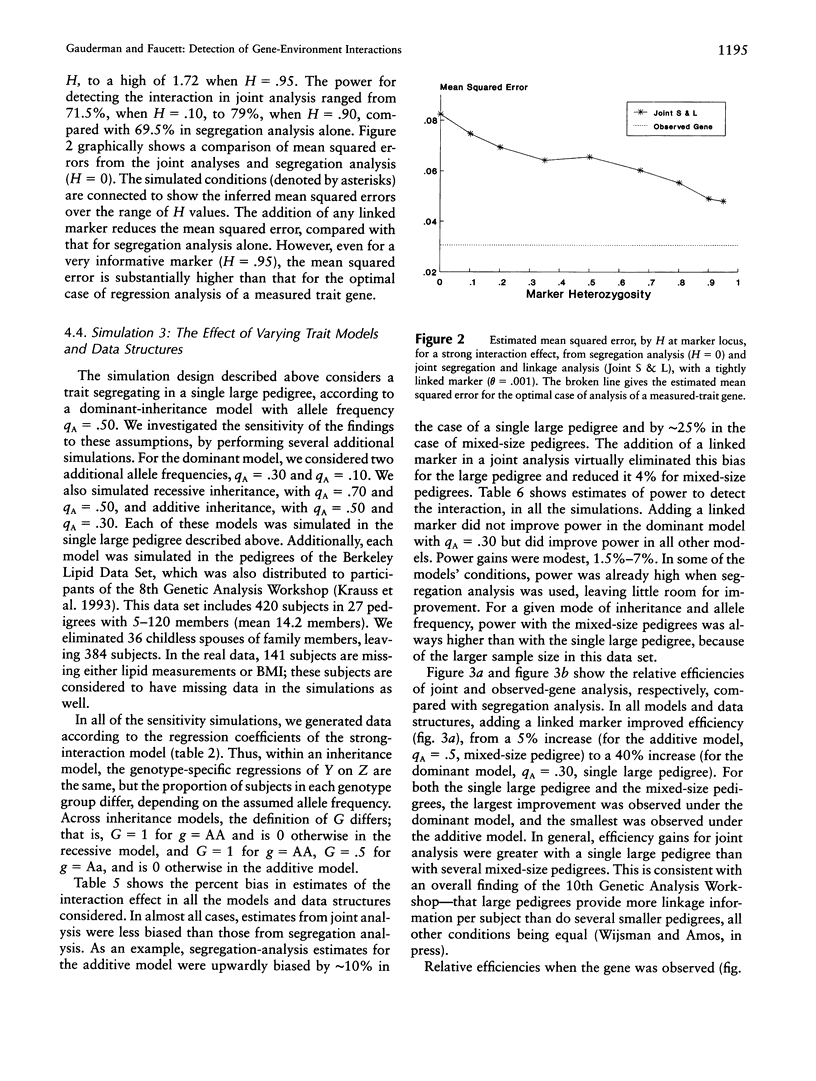
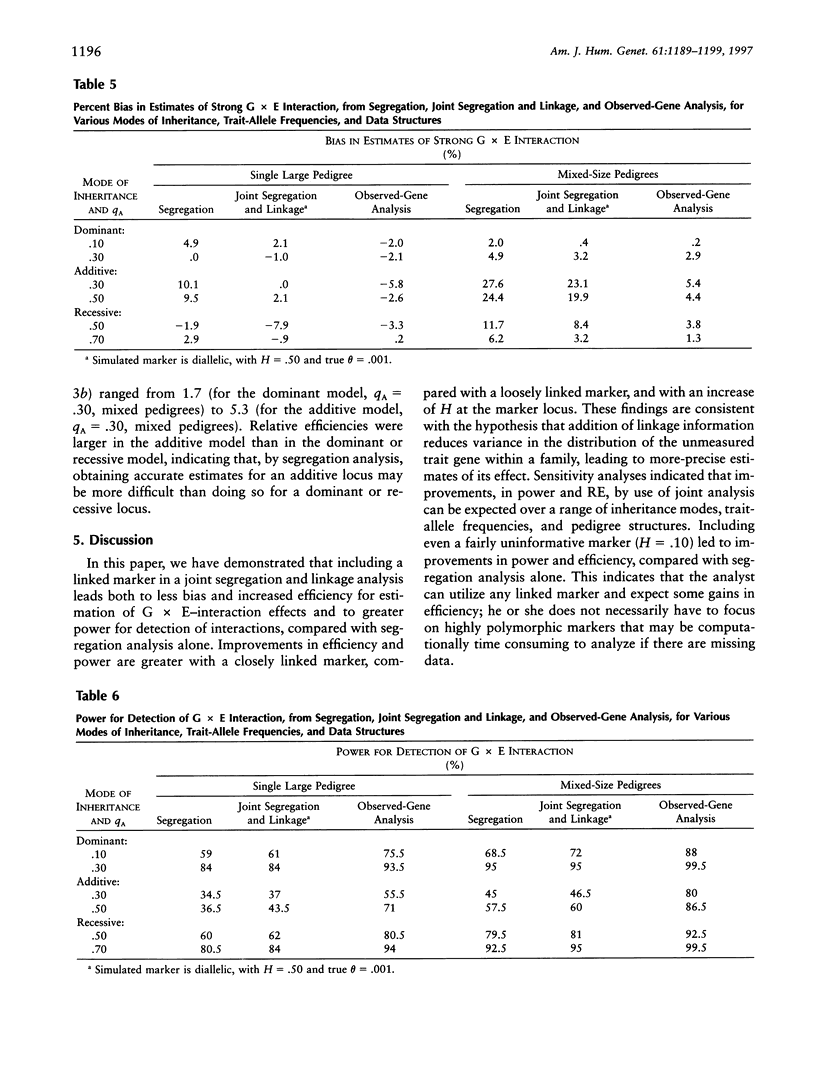
We compare approaches for analysis of gene-environment (G x E) interaction, using segregation and joint segregation and linkage analyses of a quantitative trait. Analyses of triglyceride levels in a single large pedigree demonstrate the two methods and show evidence for a significant interaction (P=.015 when segregation analysis is used; P=.006 when joint analysis is used) between a codominant major gene and body-mass index. Genotype-specific correlation coefficients, between triglyceride levels and body-mass index, estimated from the joint model are rAA=.72, rAa=.49, and raa=. 20. Several simulation studies indicate that joint segregation and linkage analysis leads to less-biased and more-efficient estimates of a G x E-interaction effect, compared with segregation analysis alone. Depending on the heterozygosity of the marker locus and its proximity to the trait locus, we found joint analysis to be as much as 70% more efficient than segregation analysis, for estimation of a G x E-interaction effect. Over a variety of parameter combinations, joint analysis also led to moderate (5%-10%) increases in power to detect the interaction. On the basis of these results, we suggest the use of combined segregation and linkage analysis for improved estimation of G x E-interaction effects when the underlying trait gene is unmeasured.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey-Wilson J. E., Elston R. C. The HGAR1 familial hypercholesterolemia pedigree. Genet Epidemiol. 1993;10(6):529–531. doi: 10.1002/gepi.1370100633. [DOI] [PubMed] [Google Scholar]
- Bailey-Wilson J. E., Wilson A. F., Bamba V. Linkage analysis in a large pedigree ascertained due to essential familial hypercholesterolemia. Genet Epidemiol. 1993;10(6):665–669. doi: 10.1002/gepi.1370100656. [DOI] [PubMed] [Google Scholar]
- Bonney G. E., Lathrop G. M., Lalouel J. M. Combined linkage and segregation analysis using regressive models. Am J Hum Genet. 1988 Jul;43(1):29–37. [PMC free article] [PubMed] [Google Scholar]
- Bonney G. E. On the statistical determination of major gene mechanisms in continuous human traits: regressive models. Am J Med Genet. 1984 Aug;18(4):731–749. doi: 10.1002/ajmg.1320180420. [DOI] [PubMed] [Google Scholar]
- Clerget-Darpoux F., Bonaïti-Pellié C., Hochez J. Effects of misspecifying genetic parameters in lod score analysis. Biometrics. 1986 Jun;42(2):393–399. [PubMed] [Google Scholar]
- Corder E. H. Weighted pairwise correlation and transmission disequilibrium in a common oligogenic disease. Genet Epidemiol. 1995;12(6):747–751. doi: 10.1002/gepi.1370120636. [DOI] [PubMed] [Google Scholar]
- Craig J. E., Rochette J., Fisher C. A., Weatherall D. J., Marc S., Lathrop G. M., Demenais F., Thein S. Dissecting the loci controlling fetal haemoglobin production on chromosomes 11p and 6q by the regressive approach. Nat Genet. 1996 Jan;12(1):58–64. doi: 10.1038/ng0196-58. [DOI] [PubMed] [Google Scholar]
- Eaves L. J. The resolution of genotype x environment interaction in segregation analysis of nuclear families. Genet Epidemiol. 1984;1(3):215–228. doi: 10.1002/gepi.1370010302. [DOI] [PubMed] [Google Scholar]
- Elston R. C., Stewart J. A general model for the genetic analysis of pedigree data. Hum Hered. 1971;21(6):523–542. doi: 10.1159/000152448. [DOI] [PubMed] [Google Scholar]
- Faucett C., Gauderman W. J., Thomas D., Ziogas A., Sobel E. Combined segregation and linkage analysis of late-onset Alzheimer's disease in Duke families using Gibbs sampling. Genet Epidemiol. 1993;10(6):489–494. doi: 10.1002/gepi.1370100627. [DOI] [PubMed] [Google Scholar]
- Gauderman W. J., Morrison J. L., Carpenter C. L., Thomas D. C. Analysis of gene-smoking interaction in lung cancer. Genet Epidemiol. 1997;14(2):199–214. doi: 10.1002/(SICI)1098-2272(1997)14:2<199::AID-GEPI8>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Gueguen R., Visvikis S., Steinmetz J., Siest G., Boerwinkle E. An analysis of genotype effects and their interactions by using the apolipoprotein E polymorphism and longitudinal data. Am J Hum Genet. 1989 Nov;45(5):793–802. [PMC free article] [PubMed] [Google Scholar]
- Guo S. W., Thompson E. A. A Monte Carlo method for combined segregation and linkage analysis. Am J Hum Genet. 1992 Nov;51(5):1111–1126. [PMC free article] [PubMed] [Google Scholar]
- Konigsberg L. W., Blangero J., Kammerer C. M., Mott G. E. Mixed model segregation analysis of LDL-C concentration with genotype-covariate interaction. Genet Epidemiol. 1991;8(2):69–80. doi: 10.1002/gepi.1370080202. [DOI] [PubMed] [Google Scholar]
- Krauss R. M., Williams P. T., Blanche P. J., Cavanaugh A., Holl L. G., Austin M. A. Lipoprotein subclasses in genetic studies: the Berkeley data set. Genet Epidemiol. 1993;10(6):523–528. doi: 10.1002/gepi.1370100632. [DOI] [PubMed] [Google Scholar]
- Lange K., Elston R. C. Extensions to pedigree analysis I. Likehood calculations for simple and complex pedigrees. Hum Hered. 1975;25(2):95–105. doi: 10.1159/000152714. [DOI] [PubMed] [Google Scholar]
- Martinez M., Abel L., Demenais F. How can maximum likelihood methods reveal candidate gene effects on a quantitative trait? Genet Epidemiol. 1995;12(6):789–794. doi: 10.1002/gepi.1370120643. [DOI] [PubMed] [Google Scholar]
- Moll P. P., Sing C. F., Lussier-Cacan S., Davignon J. An application of a model for a genotype-dependent relationship between a concomitant (age) and a quantitative trait (LDL cholesterol) in pedigree data. Genet Epidemiol. 1984;1(4):301–314. doi: 10.1002/gepi.1370010403. [DOI] [PubMed] [Google Scholar]
- Morton N. E., MacLean C. J. Analysis of family resemblance. 3. Complex segregation of quantitative traits. Am J Hum Genet. 1974 Jul;26(4):489–503. [PMC free article] [PubMed] [Google Scholar]
- Olshen A. B., Wijsman E. M. Pedigree analysis package vs. MIXD: fitting the mixed model on a large pedigree. Genet Epidemiol. 1996;13(1):91–106. doi: 10.1002/(SICI)1098-2272(1996)13:1<91::AID-GEPI8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Ottman R. Epidemiologic analysis of gene-environment interaction in twins. Genet Epidemiol. 1994;11(1):75–86. doi: 10.1002/gepi.1370110108. [DOI] [PubMed] [Google Scholar]
- Sellers T. A., Bailey-Wilson J. E., Elston R. C., Wilson A. F., Elston G. Z., Ooi W. L., Rothschild H. Evidence for mendelian inheritance in the pathogenesis of lung cancer. J Natl Cancer Inst. 1990 Aug 1;82(15):1272–1279. doi: 10.1093/jnci/82.15.1272. [DOI] [PubMed] [Google Scholar]
- Sellers T. A., Bailey-Wilson J. E., Potter J. D., Rich S. S., Rothschild H., Elston R. C. Effect of cohort differences in smoking prevalence on models of lung cancer susceptibility. Genet Epidemiol. 1992;9(4):261–271. doi: 10.1002/gepi.1370090405. [DOI] [PubMed] [Google Scholar]
- Sznajd J., Rywik S., Furberg B., Pajak A., Kurjata P., Williams O. D., Sznajderman-Ciswicka M., Misiowiec P., Irving S. H., Baczynska E. Poland and US collaborative study on cardiovascular epidemiology. II. Correlates of lipids and lipoproteins in men and women aged 35-64 years from selected Polish rural, Polish urban, and US samples. Am J Epidemiol. 1989 Sep;130(3):446–456. doi: 10.1093/oxfordjournals.aje.a115358. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Cortessis V. A Gibbs sampling approach to linkage analysis. Hum Hered. 1992;42(1):63–76. doi: 10.1159/000154046. [DOI] [PubMed] [Google Scholar]
- Tiret L., Abel L., Rakotovao R. Effect of ignoring genotype-environment interaction on segregation analysis of quantitative traits. Genet Epidemiol. 1993;10(6):581–586. doi: 10.1002/gepi.1370100642. [DOI] [PubMed] [Google Scholar]
- Tiret L., Rigat B., Visvikis S., Breda C., Corvol P., Cambien F., Soubrier F. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992 Jul;51(1):197–205. [PMC free article] [PubMed] [Google Scholar]


