Abstract
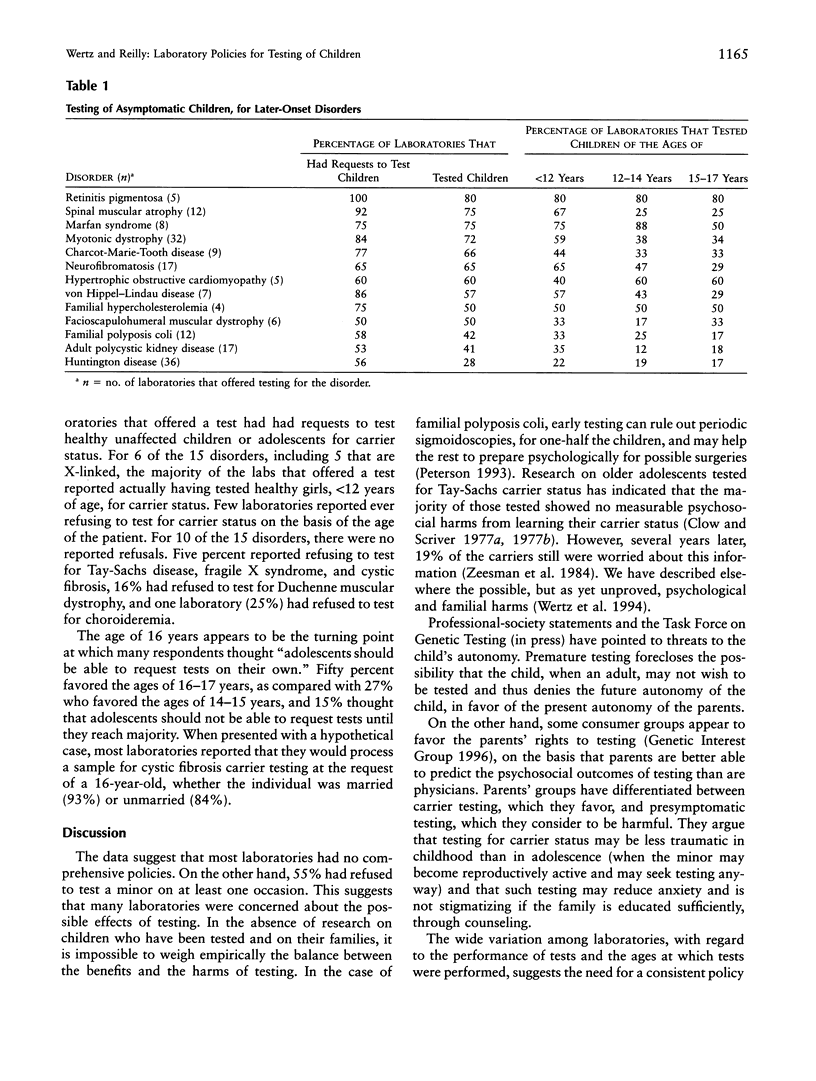
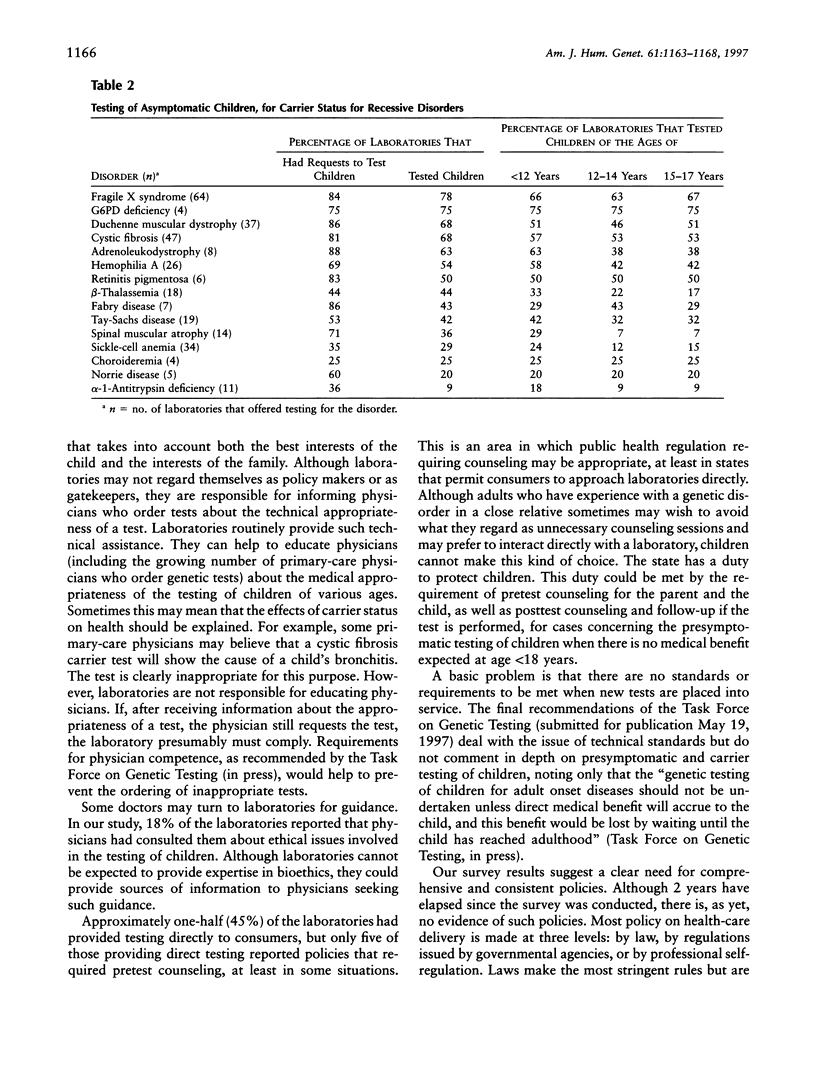
In order to discover whether laboratories have policies regarding the testing of unaffected children, we surveyed all laboratories registered with Helix, a national net-work of DNA diagnostic laboratories. Of 186 laboratories asked to respond anonymously to a four-page questionnaire, 156 (84%) replied. A screening question removed 51 laboratories that provided no clinical services. Of the remaining 105, 92% said that their requisition forms asked the person's age. Substantial minorities had policies for the testing of minors for late-onset disorders (46%), for carrier status for recessive disorders (33%), or for disorders for which the test offers no medical benefit within 3 years (33%). Most laboratories are responsive to parental requests. For 12 of 13 late-onset disorders, the majority of laboratories that offered testing had had requests to test children. The majority had tested healthy children, <12 years of age, for eight disorders. Approximately 22% had tested children, <12 years of age, for Huntington disease. Majorities had received requests to test healthy children for carrier status for 10 of 15 recessive or X-linked disorders and had tested children, <12 years of age, for 6 of these disorders, including cystic fibrosis, hemophilia A, fragile X syndrome, and Duchenne muscular dystrophy. Approximately 45% of the laboratories occasionally had provided tests directly to consumers. In view of the possibility that the harms of presymptomatic diagnoses of children sometimes may outweigh the benefits, our results suggest a need for consistent laboratory policies designed for the best interests of the child and the family.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloch M., Hayden M. R. Opinion: predictive testing for Huntington disease in childhood: challenges and implications. Am J Hum Genet. 1990 Jan;46(1):1–4. [PMC free article] [PubMed] [Google Scholar]
- Clow C. L., Scriver C. R. The adolescent copes with genetic screening: a study of Tay-Sachs screening among high-school students. Prog Clin Biol Res. 1977;18:381–393. [PubMed] [Google Scholar]
- Harper P. S., Clarke A. Should we test children for "adult" genetic diseases? Lancet. 1990 May 19;335(8699):1205–1206. doi: 10.1016/0140-6736(90)92713-r. [DOI] [PubMed] [Google Scholar]
- Petersen G. M., Francomano C., Kinzler K., Nakamura Y. Presymptomatic direct detection of adenomatous polyposis coli (APC) gene mutations in familial adenomatous polyposis. Hum Genet. 1993 May;91(4):307–311. doi: 10.1007/BF00217347. [DOI] [PubMed] [Google Scholar]
- Went L. Ethical issues policy statement on Huntington's disease molecular genetics predictive test. International Huntington Association. World Federation of Neurology. J Med Genet. 1990 Jan;27(1):34–38. doi: 10.1136/jmg.27.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz D. C., Fanos J. H., Reilly P. R. Genetic testing for children and adolescents. Who decides? JAMA. 1994 Sep 21;272(11):875–881. [PubMed] [Google Scholar]
- Zeesman S., Clow C. L., Cartier L., Scriver C. R. A private view of heterozygosity: eight-year follow-up study on carriers of the Tay-Sachs gene detected by high school screening in Montreal. Am J Med Genet. 1984 Aug;18(4):769–778. doi: 10.1002/ajmg.1320180424. [DOI] [PubMed] [Google Scholar]


