Abstract
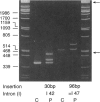
Ehlers-Danlos syndrome (EDS) type IV results from mutations in the COL3A1 gene, which encodes the constituent chains of type III procollagen. We have identified, in 33 unrelated individuals or families with EDS type IV, mutations that affect splicing, of which 30 are point mutations at splice junctions and 3 are small deletions that remove splice-junction sequences and partial exon sequences. Except for one point mutation at a donor site, which leads to partial intron inclusion, and a single base-pair substitution at an acceptor site, which gives rise to inclusion of the complete upstream intron into the mature mRNA, all mutations result in deletion of a single exon as the only splice alteration. Of the exon-skipping mutations that are due to single base substitutions, which we have identified in 28 separate individuals, only two affect the splice-acceptor site. The underrepresentation of splice acceptor-site mutations suggests that the favored consequence of 3' mutations is the use of an alternative acceptor site that creates a null allele with a premature-termination codon. The phenotypes of those mutations may differ, with respect to either their severity or their symptomatic range, from the usual presentation of EDS type IV and thus have been excluded from analysis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ala-Kokko L., Kontusaari S., Baldwin C. T., Kuivaniemi H., Prockop D. J. Structure of cDNA clones coding for the entire prepro alpha 1 (III) chain of human type III procollagen. Differences in protein structure from type I procollagen and conservation of codon preferences. Biochem J. 1989 Jun 1;260(2):509–516. doi: 10.1042/bj2600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson-Chanda V., Su M. W., Weil D., Chu M. L., Ramirez F. Cloning and analysis of the 5' portion of the human type-III procollagen gene (COL3A1). Gene. 1989 May 30;78(2):255–265. doi: 10.1016/0378-1119(89)90228-x. [DOI] [PubMed] [Google Scholar]
- Berget S. M. Exon recognition in vertebrate splicing. J Biol Chem. 1995 Feb 10;270(6):2411–2414. doi: 10.1074/jbc.270.6.2411. [DOI] [PubMed] [Google Scholar]
- Bonadio J., Holbrook K. A., Gelinas R. E., Jacob J., Byers P. H. Altered triple helical structure of type I procollagen in lethal perinatal osteogenesis imperfecta. J Biol Chem. 1985 Feb 10;260(3):1734–1742. [PubMed] [Google Scholar]
- Byers P. H., Duvic M., Atkinson M., Robinow M., Smith L. T., Krane S. M., Greally M. T., Ludman M., Matalon R., Pauker S. Ehlers-Danlos syndrome type VIIA and VIIB result from splice-junction mutations or genomic deletions that involve exon 6 in the COL1A1 and COL1A2 genes of type I collagen. Am J Med Genet. 1997 Oct 3;72(1):94–105. doi: 10.1002/(sici)1096-8628(19971003)72:1<94::aid-ajmg20>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Carter M. S., Li S., Wilkinson M. F. A splicing-dependent regulatory mechanism that detects translation signals. EMBO J. 1996 Nov 1;15(21):5965–5975. [PMC free article] [PubMed] [Google Scholar]
- Chiodo A. A., Sillence D. O., Cole W. G., Bateman J. F. Abnormal type III collagen produced by an exon-17-skipping mutation of the COL3A1 gene in Ehlers-Danlos syndrome type IV is not incorporated into the extracellular matrix. Biochem J. 1995 Nov 1;311(Pt 3):939–943. doi: 10.1042/bj3110939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cole W. G., Chiodo A. A., Lamande S. R., Janeczko R., Ramirez F., Dahl H. H., Chan D., Bateman J. F. A base substitution at a splice site in the COL3A1 gene causes exon skipping and generates abnormal type III procollagen in a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1990 Oct 5;265(28):17070–17077. [PubMed] [Google Scholar]
- Del Gatto F., Gesnel M. C., Breathnach R. The exon sequence TAGG can inhibit splicing. Nucleic Acids Res. 1996 Jun 1;24(11):2017–2021. doi: 10.1093/nar/24.11.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Estes P. A., Cooke N. E., Liebhaber S. A. A native RNA secondary structure controls alternative splice-site selection and generates two human growth hormone isoforms. J Biol Chem. 1992 Jul 25;267(21):14902–14908. [PubMed] [Google Scholar]
- Hawkins J. D. A survey on intron and exon lengths. Nucleic Acids Res. 1988 Nov 11;16(21):9893–9908. doi: 10.1093/nar/16.21.9893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnane J., Priebe C., Caty M., Kuppermann N. Perforation of the colon in an adolescent girl. Pediatr Emerg Care. 1995 Aug;11(4):230–232. doi: 10.1097/00006565-199508000-00010. [DOI] [PubMed] [Google Scholar]
- Kontusaari S., Tromp G., Kuivaniemi H., Ladda R. L., Prockop D. J. Inheritance of an RNA splicing mutation (G+ 1 IVS20) in the type III procollagen gene (COL3A1) in a family having aortic aneurysms and easy bruisability: phenotypic overlap between familial arterial aneurysms and Ehlers-Danlos syndrome type IV. Am J Hum Genet. 1990 Jul;47(1):112–120. [PMC free article] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Kontusaari S., Tromp G., Zhao M. J., Sabol C., Prockop D. J. Identical G+1 to A mutations in three different introns of the type III procollagen gene (COL3A1) produce different patterns of RNA splicing in three variants of Ehlers-Danlos syndrome. IV. An explanation for exon skipping some mutations and not others. J Biol Chem. 1990 Jul 15;265(20):12067–12074. [PubMed] [Google Scholar]
- Kuivaniemi H., Prockop D. J., Wu Y., Madhatheri S. L., Kleinert C., Earley J. J., Jokinen A., Stolle C., Majamaa K., Myllylä V. V. Exclusion of mutations in the gene for type III collagen (COL3A1) as a common cause of intracranial aneurysms or cervical artery dissections: results from sequence analysis of the coding sequences of type III collagen from 55 unrelated patients. Neurology. 1993 Dec;43(12):2652–2658. doi: 10.1212/wnl.43.12.2652. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Bergfeld W. F., Kay M., Helm T. N. Ehlers-Danlos syndrome type IV: a single base substitution of the last nucleotide of exon 34 in COL3A1 leads to exon skipping. J Invest Dermatol. 1995 Sep;105(3):352–356. doi: 10.1111/1523-1747.ep12320704. [DOI] [PubMed] [Google Scholar]
- Kuivaniemi H., Tromp G., Prockop D. J. Mutations in fibrillar collagens (types I, II, III, and XI), fibril-associated collagen (type IX), and network-forming collagen (type X) cause a spectrum of diseases of bone, cartilage, and blood vessels. Hum Mutat. 1997;9(4):300–315. doi: 10.1002/(SICI)1098-1004(1997)9:4<300::AID-HUMU2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Lee B., Vitale E., Superti-Furga A., Steinmann B., Ramirez F. G to T transversion at position +5 of a splice donor site causes skipping of the preceding exon in the type III procollagen transcripts of a patient with Ehlers-Danlos syndrome type IV. J Biol Chem. 1991 Mar 15;266(8):5256–5259. [PubMed] [Google Scholar]
- Liu X., Wu H., Byrne M., Krane S., Jaenisch R. Type III collagen is crucial for collagen I fibrillogenesis and for normal cardiovascular development. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1852–1856. doi: 10.1073/pnas.94.5.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd J., Narcisi P., Richards A., Pope F. M. A T+6 to C+6 mutation in the donor splice site of COL3A1 IVS7 causes exon skipping and results in Ehlers-Danlos syndrome type IV. J Med Genet. 1993 May;30(5):376–380. doi: 10.1136/jmg.30.5.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan A. M., McCaw P. S., Crispino J. D., Sharp P. A. SC35-mediated reconstitution of splicing in U2AF-depleted nuclear extract. Proc Natl Acad Sci U S A. 1997 Jan 7;94(1):133–136. doi: 10.1073/pnas.94.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquat L. E. When cells stop making sense: effects of nonsense codons on RNA metabolism in vertebrate cells. RNA. 1995 Jul;1(5):453–465. [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Peterson M. L., Bryman M. B., Peiter M., Cowan C. Exon size affects competition between splicing and cleavage-polyadenylation in the immunoglobulin mu gene. Mol Cell Biol. 1994 Jan;14(1):77–86. doi: 10.1128/mcb.14.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope F. M., Narcisi P., Nicholls A. C., Germaine D., Pals G., Richards A. J. COL3A1 mutations cause variable clinical phenotypes including acrogeria and vascular rupture. Br J Dermatol. 1996 Aug;135(2):163–181. [PubMed] [Google Scholar]
- Redford-Badwal D. A., Stover M. L., Valli M., McKinstry M. B., Rowe D. W. Nuclear retention of COL1A1 messenger RNA identifies null alleles causing mild osteogenesis imperfecta. J Clin Invest. 1996 Feb 15;97(4):1035–1040. doi: 10.1172/JCI118495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards A. J., Narcisi P., Ferguson C., Cobben J. M., Pope F. M. Two new mutations affecting the donor splice site of COL3A1 IVS37 and causing skipping of exon 37 in patients with Ehlers-Danlos syndrome type IV. Hum Mol Genet. 1994 Oct;3(10):1901–1902. doi: 10.1093/hmg/3.10.1901. [DOI] [PubMed] [Google Scholar]
- Robberson B. L., Cote G. J., Berget S. M. Exon definition may facilitate splice site selection in RNAs with multiple exons. Mol Cell Biol. 1990 Jan;10(1):84–94. doi: 10.1128/mcb.10.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sirand-Pugnet P., Durosay P., Brody E., Marie J. An intronic (A/U)GGG repeat enhances the splicing of an alternative intron of the chicken beta-tropomyosin pre-mRNA. Nucleic Acids Res. 1995 Sep 11;23(17):3501–3507. doi: 10.1093/nar/23.17.3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. T., Schwarze U., Goldstein J., Byers P. H. Mutations in the COL3A1 gene result in the Ehlers-Danlos syndrome type IV and alterations in the size and distribution of the major collagen fibrils of the dermis. J Invest Dermatol. 1997 Mar;108(3):241–247. doi: 10.1111/1523-1747.ep12286441. [DOI] [PubMed] [Google Scholar]
- Sterner D. A., Berget S. M. In vivo recognition of a vertebrate mini-exon as an exon-intron-exon unit. Mol Cell Biol. 1993 May;13(5):2677–2687. doi: 10.1128/mcb.13.5.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover M. L., Primorac D., Liu S. C., McKinstry M. B., Rowe D. W. Defective splicing of mRNA from one COL1A1 allele of type I collagen in nondeforming (type I) osteogenesis imperfecta. J Clin Invest. 1993 Oct;92(4):1994–2002. doi: 10.1172/JCI116794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakker-Varia S., Anderson D. W., Kuivaniemi H., Tromp G., Shin H. G., van der Rest M., Glorieux F. H., Ala-Kokko L., Stolle C. A. Aberrant splicing of the type III procollagen mRNA leads to intracellular degradation of the protein in a patient with Ehlers-Danlos type IV. Hum Mutat. 1995;6(2):116–125. doi: 10.1002/humu.1380060204. [DOI] [PubMed] [Google Scholar]
- Tromp G., Wu Y., Prockop D. J., Madhatheri S. L., Kleinert C., Earley J. J., Zhuang J., Norrgård O., Darling R. C., Abbott W. M. Sequencing of cDNA from 50 unrelated patients reveals that mutations in the triple-helical domain of type III procollagen are an infrequent cause of aortic aneurysms. J Clin Invest. 1993 Jun;91(6):2539–2545. doi: 10.1172/JCI116490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Byers P. H., Schwartz R. C., Chu M. L., Weil D., Pepe G., Cassidy S. B., Ramirez F. Ehlers-Danlos syndrome type IV: cosegregation of the phenotype to a COL3A1 allele of type III procollagen. Hum Genet. 1986 Sep;74(1):41–46. doi: 10.1007/BF00278783. [DOI] [PubMed] [Google Scholar]
- Valcárcel J., Gaur R. K., Singh R., Green M. R. Interaction of U2AF65 RS region with pre-mRNA branch point and promotion of base pairing with U2 snRNA [corrected]. Science. 1996 Sep 20;273(5282):1706–1709. doi: 10.1126/science.273.5282.1706. [DOI] [PubMed] [Google Scholar]
- Wang J., Takagaki Y., Manley J. L. Targeted disruption of an essential vertebrate gene: ASF/SF2 is required for cell viability. Genes Dev. 1996 Oct 15;10(20):2588–2599. doi: 10.1101/gad.10.20.2588. [DOI] [PubMed] [Google Scholar]
- Weinbaum P. J., Cassidy S. B., Campbell W. A., Rickles F. R., Vintzileos A. M., Nochimson D. J., Tsipouras P. Pregnancy management and successful outcome of Ehlers-Danlos syndrome type IV. Am J Perinatol. 1987 Apr;4(2):134–137. doi: 10.1055/s-2007-999756. [DOI] [PubMed] [Google Scholar]
- Willing M. C., Deschenes S. P., Scott D. A., Byers P. H., Slayton R. L., Pitts S. H., Arikat H., Roberts E. J. Osteogenesis imperfecta type I: molecular heterogeneity for COL1A1 null alleles of type I collagen. Am J Hum Genet. 1994 Oct;55(4):638–647. [PMC free article] [PubMed] [Google Scholar]
- Willing M. C., Deschenes S. P., Slayton R. L., Roberts E. J. Premature chain termination is a unifying mechanism for COL1A1 null alleles in osteogenesis imperfecta type I cell strains. Am J Hum Genet. 1996 Oct;59(4):799–809. [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Kuivaniemi H., Tromp G., Strobel D., Romanic A. M., Prockop D. J. Temperature sensitivity of aberrant RNA splicing with a mutation in the G+5 position of intron 37 of the gene for type III procollagen from a patient with Ehlers-Danlos syndrome type IV. Hum Mutat. 1993;2(1):28–36. doi: 10.1002/humu.1380020106. [DOI] [PubMed] [Google Scholar]
- van Oers C. C., Adema G. J., Zandberg H., Moen T. C., Baas P. D. Two different sequence elements within exon 4 are necessary for calcitonin-specific splicing of the human calcitonin/calcitonin gene-related peptide I pre-mRNA. Mol Cell Biol. 1994 Feb;14(2):951–960. doi: 10.1128/mcb.14.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]