Abstract
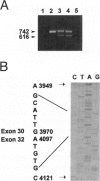
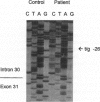
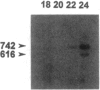
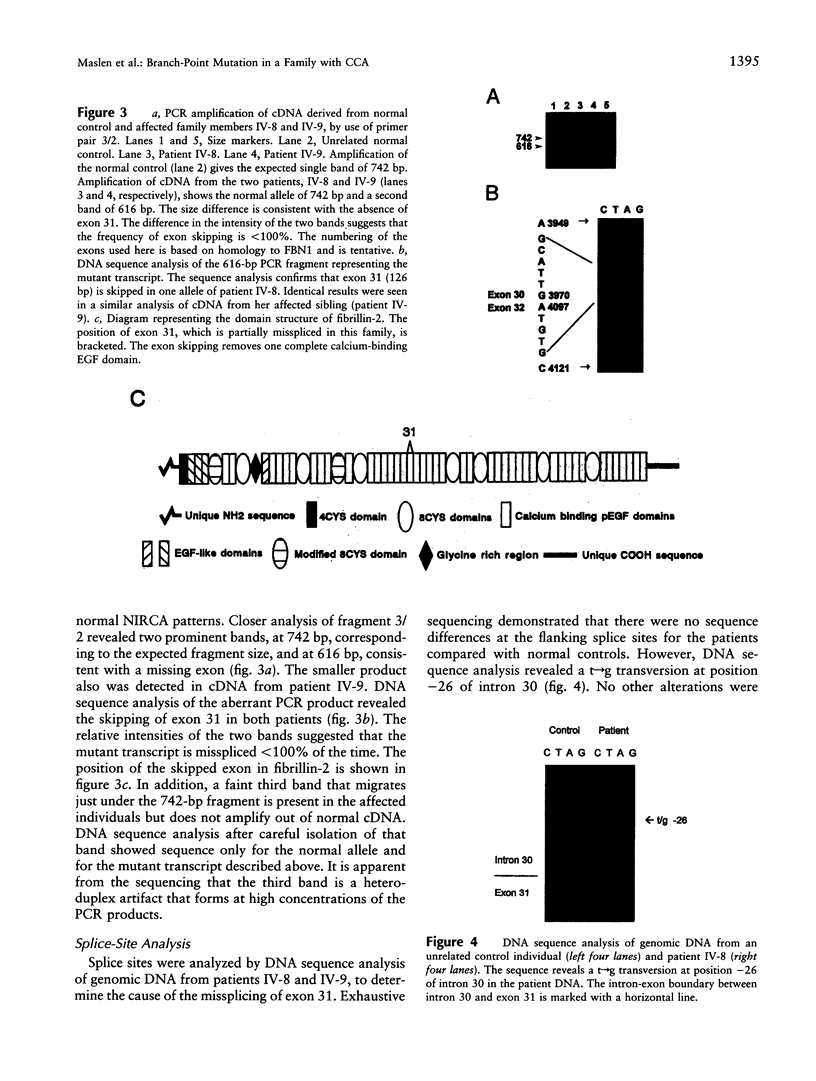
Congenital contractural arachnodactyly (CCA) is an autosomal dominant disorder that is phenotypically similar to but genetically distinct from Marfan syndrome. Genetic-linkage analysis has implicated the fibrillin-2 gene (FBN2) as the CCA locus. Mutation analysis of two isolated CCA patients revealed missense mutations, indicating that defects in FBN2 may be responsible for this disorder. However, cosegregation of a mutant allele with the disease phenotype has not yet been established. We have investigated the primary cause of CCA in a large well-characterized kindred with five generations comprising 18 affected individuals. Previous studies demonstrated linkage of this family's CCA phenotype to FBN2. Mutation analysis of cDNA derived from the proband and her affected brother, using a nonisotopic RNase cleavage assay, revealed the partial skipping of exon 31. Approximately 25% mutant transcript is produced, which is apparently sufficient to cause a CCA phenotype. Sequence analysis of genomic DNA revealed an unusual base composition for intron 30 and identified the mutation, a g-26t transversion, in the vicinity of the splicing branch-point site in intron 30. Genomic DNA from 30 additional family members, both affected and unaffected, then was analyzed for the mutation. The results clearly demonstrate cosegregation of the branch-point mutation with the CCA phenotype. This is the first report of a CCA mutation in a multiplex family, unequivocally establishing that mutation in FBN2 are responsible for the CCA phenotype. In addition, branch-point mutations only very rarely have been associated with human disease, suggesting that the unusual composition of this intron influences splicing stability.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Koch S., Camerini-Otero R. D. Cardiovascular findings in congenital contractural arachnodactyly: report of an affected kindred. Am J Med Genet. 1984 Jun;18(2):265–271. doi: 10.1002/ajmg.1320180210. [DOI] [PubMed] [Google Scholar]
- Beals R. K., Hecht F. Congenital contractural arachnodactyly. A heritable disorder of connective tissue. J Bone Joint Surg Am. 1971 Jul;53(5):987–993. [PubMed] [Google Scholar]
- Bottema C. D., Sarkar G., Cassady J. D., Ii S., Dutton C. M., Sommer S. S. Polymerase chain reaction amplification of specific alleles: a general method of detection of mutations, polymorphisms, and haplotypes. Methods Enzymol. 1993;218:388–402. doi: 10.1016/0076-6879(93)18031-7. [DOI] [PubMed] [Google Scholar]
- Fülöp C., Cs-Szabó G., Glant T. T. Species-specific alternative splicing of the epidermal growth factor-like domain 1 of cartilage aggrecan. Biochem J. 1996 Nov 1;319(Pt 3):935–940. doi: 10.1042/bj3190935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goguel V., Rosbash M. Splice site choice and splicing efficiency are positively influenced by pre-mRNA intramolecular base pairing in yeast. Cell. 1993 Mar 26;72(6):893–901. doi: 10.1016/0092-8674(93)90578-e. [DOI] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Hecht F., Beals R. K. "New" syndrome of congenital contractural arachnodactyly originally described by Marfan in 1896. Pediatrics. 1972 Apr;49(4):574–579. [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Lee B., Godfrey M., Vitale E., Hori H., Mattei M. G., Sarfarazi M., Tsipouras P., Ramirez F., Hollister D. W. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991 Jul 25;352(6333):330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Nijbroek G., Sood S., McIntosh I., Francomano C. A., Bull E., Pereira L., Ramirez F., Pyeritz R. E., Dietz H. C. Fifteen novel FBN1 mutations causing Marfan syndrome detected by heteroduplex analysis of genomic amplicons. Am J Hum Genet. 1995 Jul;57(1):8–21. [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Gotoh Y. Signals for the selection of a splice site in pre-mRNA. Computer analysis of splice junction sequences and like sequences. J Mol Biol. 1987 May 20;195(2):247–259. doi: 10.1016/0022-2836(87)90647-4. [DOI] [PubMed] [Google Scholar]
- Putnam E. A., Cho M., Zinn A. B., Towbin J. A., Byers P. H., Milewicz D. M. Delineation of the Marfan phenotype associated with mutations in exons 23-32 of the FBN1 gene. Am J Med Genet. 1996 Mar 29;62(3):233–242. doi: 10.1002/(SICI)1096-8628(19960329)62:3<233::AID-AJMG7>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Putnam E. A., Zhang H., Ramirez F., Milewicz D. M. Fibrillin-2 (FBN2) mutations result in the Marfan-like disorder, congenital contractural arachnodactyly. Nat Genet. 1995 Dec;11(4):456–458. doi: 10.1038/ng1295-456. [DOI] [PubMed] [Google Scholar]
- Raben N., Nichols R. C., Martiniuk F., Plotz P. H. A model of mRNA splicing in adult lysosomal storage disease (glycogenosis type II). Hum Mol Genet. 1996 Jul;5(7):995–1000. doi: 10.1093/hmg/5.7.995. [DOI] [PubMed] [Google Scholar]
- Ramirez F. Fibrillln mutations in Marfan syndrome and related phenotypes. Curr Opin Genet Dev. 1996 Jun;6(3):309–315. doi: 10.1016/s0959-437x(96)80007-4. [DOI] [PubMed] [Google Scholar]
- Ramos Arroyo M. A., Weaver D. D., Beals R. K. Congenital contractural arachnodactyly. Report of four additional families and review of literature. Clin Genet. 1985 Jun;27(6):570–581. doi: 10.1111/j.1399-0004.1985.tb02042.x. [DOI] [PubMed] [Google Scholar]
- Tsipouras P., Del Mastro R., Sarfarazi M., Lee B., Vitale E., Child A. H., Godfrey M., Devereux R. B., Hewett D., Steinmann B. Genetic linkage of the Marfan syndrome, ectopia lentis, and congenital contractural arachnodactyly to the fibrillin genes on chromosomes 15 and 5. The International Marfan Syndrome Collaborative Study. N Engl J Med. 1992 Apr 2;326(14):905–909. doi: 10.1056/NEJM199204023261401. [DOI] [PubMed] [Google Scholar]
- Viljoen D. Congenital contractural arachnodactyly (Beals syndrome). J Med Genet. 1994 Aug;31(8):640–643. doi: 10.1136/jmg.31.8.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Clericuzio C. L., Godfrey M. Familial occurrence of typical and severe lethal congenital contractural arachnodactyly caused by missplicing of exon 34 of fibrillin-2. Am J Hum Genet. 1996 Nov;59(5):1027–1034. [PMC free article] [PubMed] [Google Scholar]
- Wang M., Price C., Han J., Cisler J., Imaizumi K., Van Thienen M. N., DePaepe A., Godfrey M. Recurrent mis-splicing of fibrillin exon 32 in two patients with neonatal Marfan syndrome. Hum Mol Genet. 1995 Apr;4(4):607–613. doi: 10.1093/hmg/4.4.607. [DOI] [PubMed] [Google Scholar]
- Webb J. C., Patel D. D., Shoulders C. C., Knight B. L., Soutar A. K. Genetic variation at a splicing branch point in intron 9 of the low density lipoprotein (LDL)-receptor gene: a rare mutation that disrupts mRNA splicing in a patient with familial hypercholesterolaemia and a common polymorphism. Hum Mol Genet. 1996 Sep;5(9):1325–1331. doi: 10.1093/hmg/5.9.1325. [DOI] [PubMed] [Google Scholar]
- Zhang H., Apfelroth S. D., Hu W., Davis E. C., Sanguineti C., Bonadio J., Mecham R. P., Ramirez F. Structure and expression of fibrillin-2, a novel microfibrillar component preferentially located in elastic matrices. J Cell Biol. 1994 Mar;124(5):855–863. doi: 10.1083/jcb.124.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]