Abstract
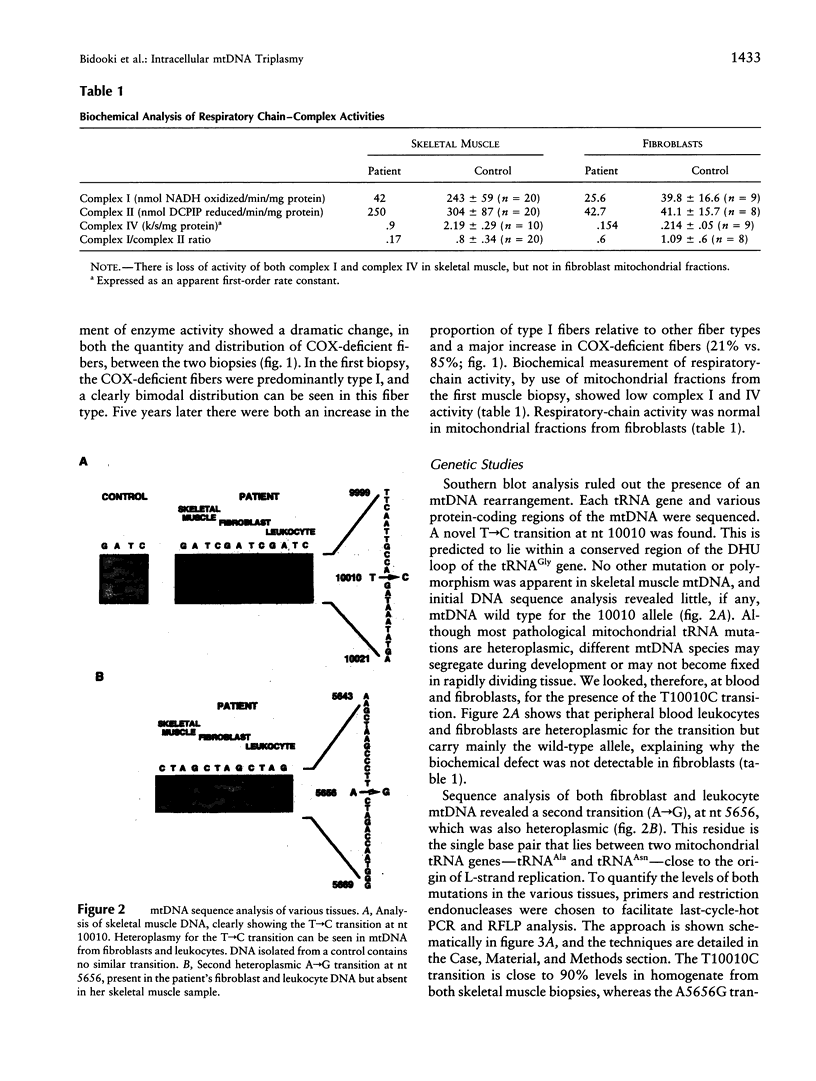
We report the clinical, biochemical, and genetic investigation of a patient with a severe mitochondrial encephalomyopathy. Genetic studies identified a novel, heteroplasmic tRNA mutation at nt 10010. This T-->C transition is located in the DHU loop of mitochondrial tRNA(Gly). In skeletal muscle, it was present at lower levels in cytochrome c oxidase (COX)-normal (87.2% +/- 11%) compared with COX-deficient fibers (97.3% +/- 2.6%); it was found in skin fibroblasts and blood cells, but at lower levels of heteroplasmy (15% +/- 6% and 17% +/- 10%, respectively). A second, heteroplasmic transition (A-->G), at nt 5656, showed a different distribution than the tRNA(Gly) mutation, with very low levels in skeletal muscle (< 3%) but higher levels in blood (22.7% +/- 3%) and skin fibroblasts (21% +/- 2%). These transitions were followed both in vivo, by repeat biopsy and blood sampling, and in vitro, by establishing primary cultures of myoblasts and skin fibroblasts. Repeat muscle biopsy showed a dramatic increase in COX-deficient fibers, but not of the tRNAGly mutation. Indeed, no significant change in heteroplasmy was measured for either substitution in muscle or blood. In vitro analysis gave very different results. The T10010C was not found in cultured myoblasts, even at early passage. In uncloned fibroblasts, the T10010C was stable (approximately 10%) for several passages but then gradually was lost. In contrast, the A5656G rose progressively from 27% to 91%. In cloned fibroblasts, different combinations of both base-pair changes and wild type could be identified, confirming the presence of clonal, intracellular triplasmy.
Full text
PDF

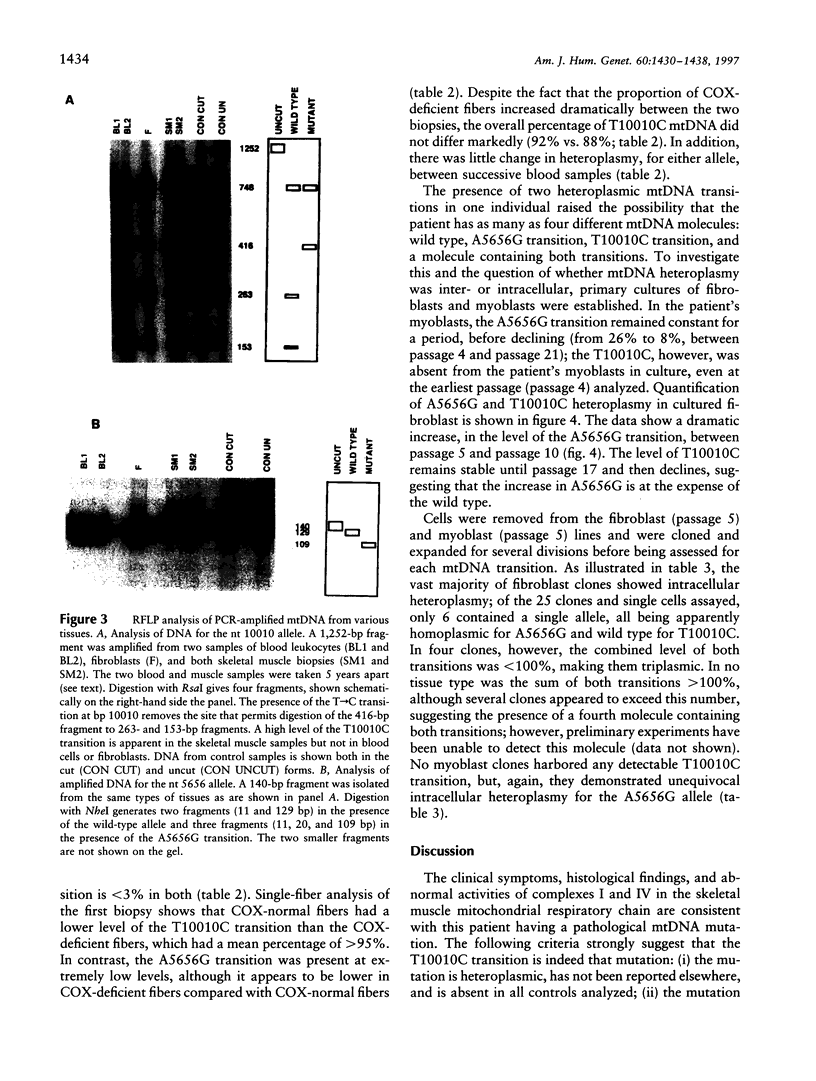
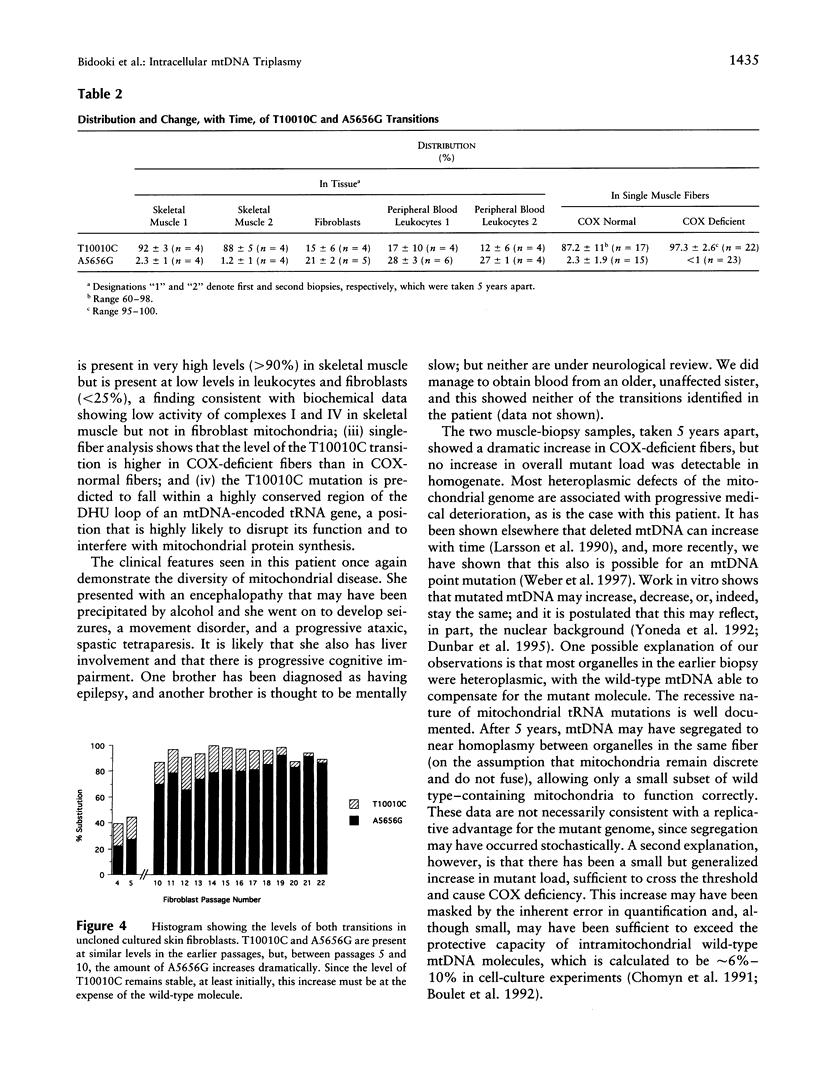
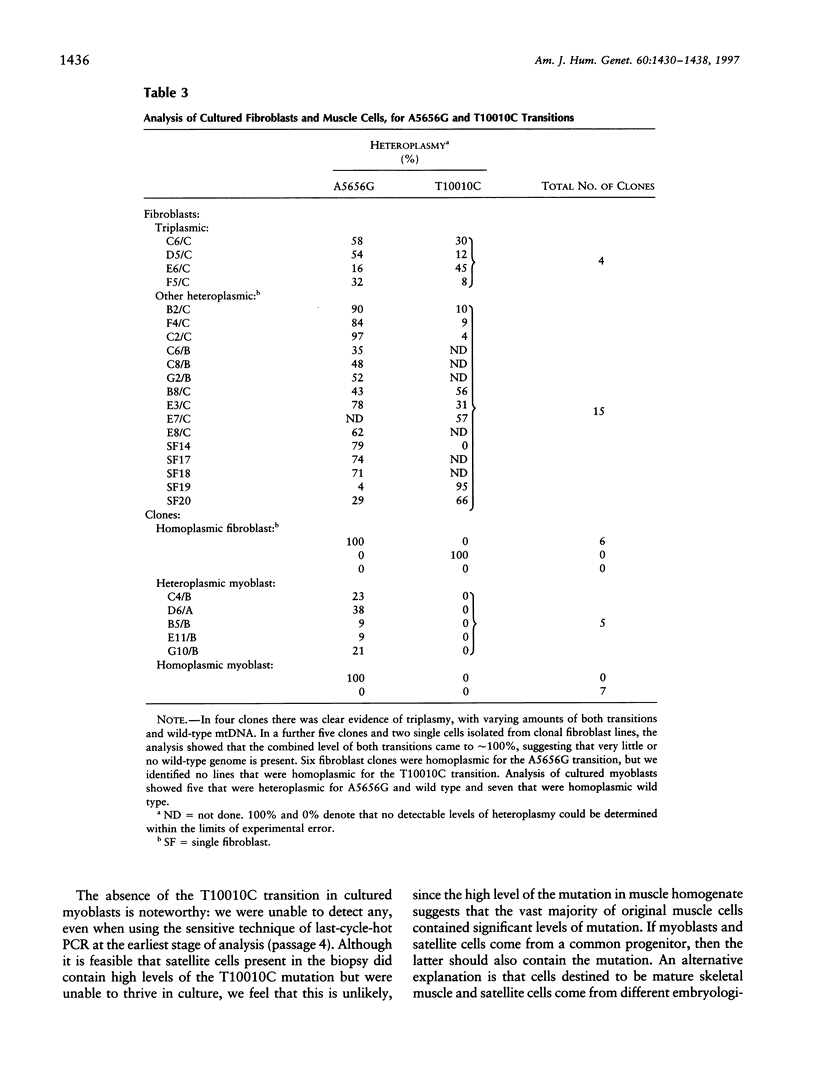


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Bendall K. E., Sykes B. C. Length heteroplasmy in the first hypervariable segment of the human mtDNA control region. Am J Hum Genet. 1995 Aug;57(2):248–256. [PMC free article] [PubMed] [Google Scholar]
- Bindoff L. A., Desnuelle C., Birch-Machin M. A., Pellissier J. F., Serratrice G., Dravet C., Bureau M., Howell N., Turnbull D. M. Multiple defects of the mitochondrial respiratory chain in a mitochondrial encephalopathy (MERRF): a clinical, biochemical and molecular study. J Neurol Sci. 1991 Mar;102(1):17–24. doi: 10.1016/0022-510x(91)90088-o. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet L., Karpati G., Shoubridge E. A. Distribution and threshold expression of the tRNA(Lys) mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1992 Dec;51(6):1187–1200. [PMC free article] [PubMed] [Google Scholar]
- Brockington M., Sweeney M. G., Hammans S. R., Morgan-Hughes J. A., Harding A. E. A tandem duplication in the D-loop of human mitochondrial DNA is associated with deletions in mitochondrial myopathies. Nat Genet. 1993 May;4(1):67–71. doi: 10.1038/ng0593-67. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunbar D. R., Moonie P. A., Jacobs H. T., Holt I. J. Different cellular backgrounds confer a marked advantage to either mutant or wild-type mitochondrial genomes. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6562–6566. doi: 10.1073/pnas.92.14.6562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu K., Hartlen R., Johns T., Genge A., Karpati G., Shoubridge E. A. A novel heteroplasmic tRNAleu(CUN) mtDNA point mutation in a sporadic patient with mitochondrial encephalomyopathy segregates rapidly in skeletal muscle and suggests an approach to therapy. Hum Mol Genet. 1996 Nov;5(11):1835–1840. doi: 10.1093/hmg/5.11.1835. [DOI] [PubMed] [Google Scholar]
- Howell N., Kubacka I., Mackey D. A. How rapidly does the human mitochondrial genome evolve? Am J Hum Genet. 1996 Sep;59(3):501–509. [PMC free article] [PubMed] [Google Scholar]
- Jackson M. J., Bindoff L. A., Weber K., Wilson J. N., Ince P., Alberti K. G., Turnbull D. M. Biochemical and molecular studies of mitochondrial function in diabetes insipidus, diabetes mellitus, optic atrophy, and deafness. Diabetes Care. 1994 Jul;17(7):728–733. doi: 10.2337/diacare.17.7.728. [DOI] [PubMed] [Google Scholar]
- Jenuth J. P., Peterson A. C., Fu K., Shoubridge E. A. Random genetic drift in the female germline explains the rapid segregation of mammalian mitochondrial DNA. Nat Genet. 1996 Oct;14(2):146–151. doi: 10.1038/ng1096-146. [DOI] [PubMed] [Google Scholar]
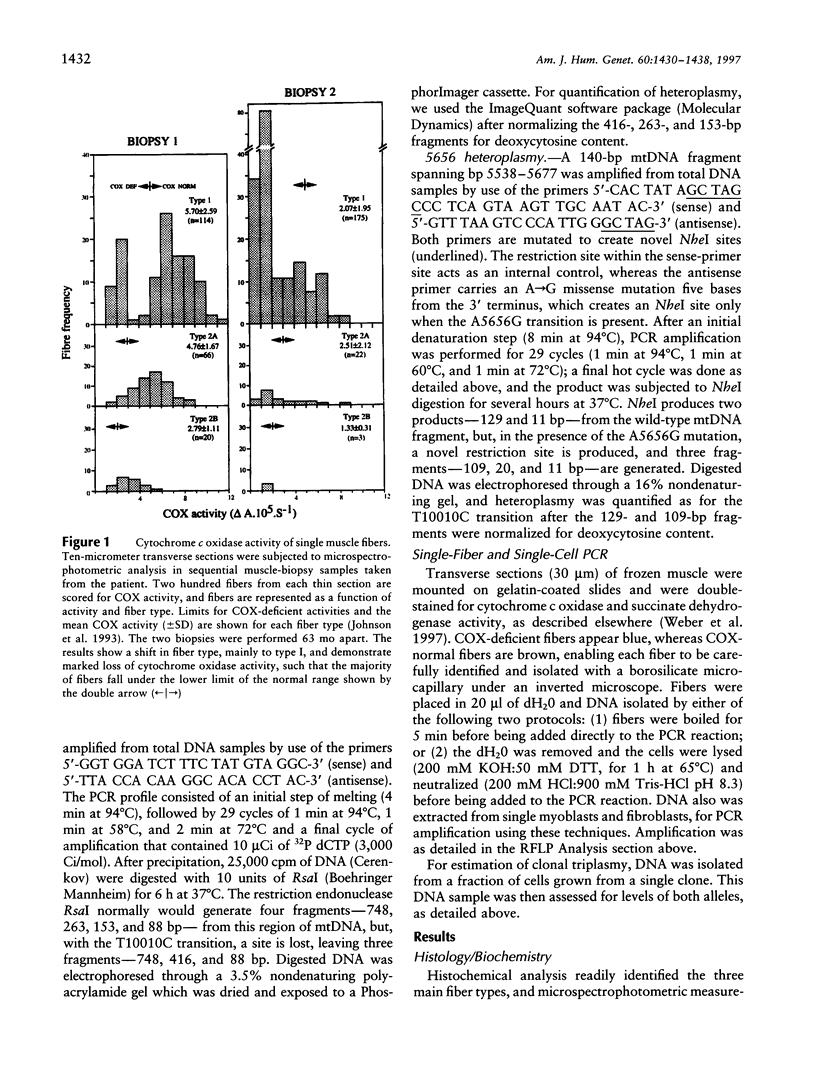
- Johnson M. A., Bindoff L. A., Turnbull D. M. Cytochrome c oxidase activity in single muscle fibers: assay techniques and diagnostic applications. Ann Neurol. 1993 Jan;33(1):28–35. doi: 10.1002/ana.410330106. [DOI] [PubMed] [Google Scholar]
- Kadenbach B., Münscher C., Frank V., Müller-Höcker J., Napiwotzki J. Human aging is associated with stochastic somatic mutations of mitochondrial DNA. Mutat Res. 1995 Oct;338(1-6):161–172. doi: 10.1016/0921-8734(95)00021-w. [DOI] [PubMed] [Google Scholar]
- Larsson N. G., Holme E., Kristiansson B., Oldfors A., Tulinius M. Progressive increase of the mutated mitochondrial DNA fraction in Kearns-Sayre syndrome. Pediatr Res. 1990 Aug;28(2):131–136. doi: 10.1203/00006450-199008000-00011. [DOI] [PubMed] [Google Scholar]
- Lowerson S. A., Taylor L., Briggs H. L., Turnbull D. M. Measurement of the activity of individual respiratory chain complexes in isolated fibroblast mitochondria. Anal Biochem. 1992 Sep;205(2):372–374. doi: 10.1016/0003-2697(92)90453-e. [DOI] [PubMed] [Google Scholar]
- Morten K. J., Poulton J., Sykes B. Multiple independent occurrence of the 3243 mutation in mitochondrial tRNA(leuUUR) in patients with the MELAS phenotype. Hum Mol Genet. 1995 Sep;4(9):1689–1691. doi: 10.1093/hmg/4.9.1689. [DOI] [PubMed] [Google Scholar]
- Ohno K., Yamamoto M., Engel A. G., Harper C. M., Roberts L. R., Tan G. H., Fatourechi V. MELAS- and Kearns-Sayre-type co-mutation [corrected] with myopathy and autoimmune polyendocrinopathy. Ann Neurol. 1996 Jun;39(6):761–766. doi: 10.1002/ana.410390612. [DOI] [PubMed] [Google Scholar]
- Thomas A. W., Edwards A., Sherratt E. J., Majid A., Gagg J., Alcolado J. C. Molecular scanning of candidate mitochondrial tRNA genes in type 2 (non-insulin dependent) diabetes mellitus. J Med Genet. 1996 Mar;33(3):253–255. doi: 10.1136/jmg.33.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan B., Padua R. A., Campbell C. Mammalian mitochondria possess homologous DNA recombination activity. J Biol Chem. 1996 Nov 1;271(44):27536–27543. doi: 10.1074/jbc.271.44.27536. [DOI] [PubMed] [Google Scholar]
- Wallace D. C. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- Weber K., Wilson J. N., Taylor L., Brierley E., Johnson M. A., Turnbull D. M., Bindoff L. A. A new mtDNA mutation showing accumulation with time and restriction to skeletal muscle. Am J Hum Genet. 1997 Feb;60(2):373–380. [PMC free article] [PubMed] [Google Scholar]
- Yoneda M., Chomyn A., Martinuzzi A., Hurko O., Attardi G. Marked replicative advantage of human mtDNA carrying a point mutation that causes the MELAS encephalomyopathy. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11164–11168. doi: 10.1073/pnas.89.23.11164. [DOI] [PMC free article] [PubMed] [Google Scholar]