Abstract
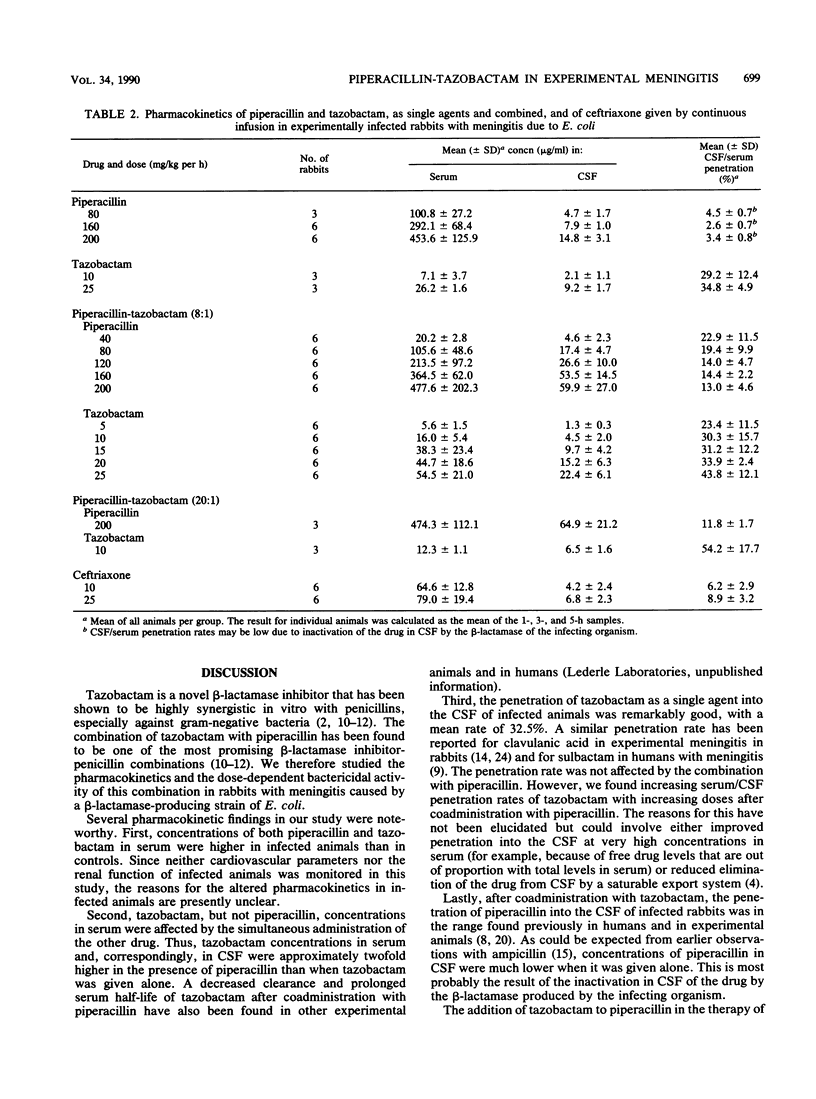
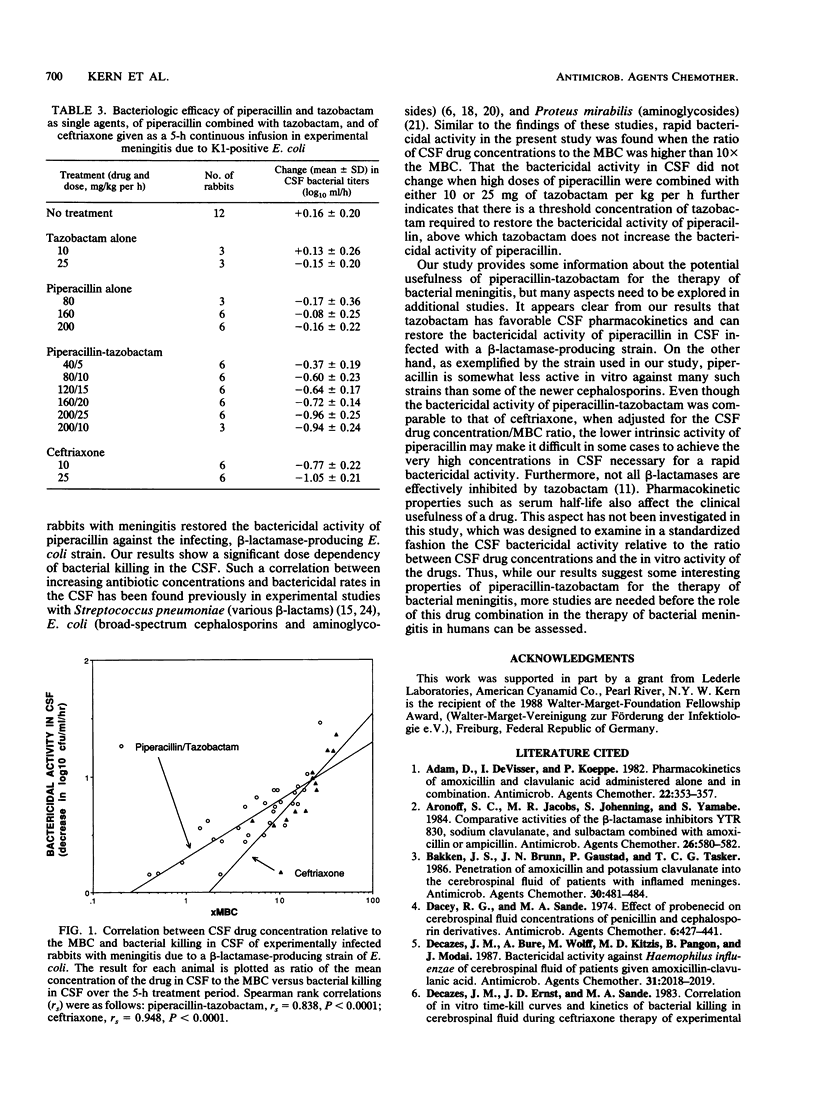
We evaluated the pharmacokinetics and therapeutic efficacy of piperacillin combined with tazobactam, a novel beta-lactamase inhibitor, in experimental meningitis due to a beta-lactamase-producing strain of K1-positive Escherichia coli. Different doses of piperacillin and tazobactam, as single agents and combined (8:1 ratio; dosage range, 40/5 to 200/25 mg/kg per h), and of ceftriaxone were given to experimentally infected rabbits by intravenous bolus injection followed by a 5-h constant infusion. The mean (+/- standard deviation) rates for penetration into the cerebrospinal fluid of infected animals after coadministration of both drugs were 16.6 +/- 8.4% for piperacillin and 32.5 +/- 12.6% for tazobactam. Compared with either agent alone, combination treatment resulted in significantly better bactericidal activity in the cerebrospinal fluid. The bactericidal activity of piperacillin-tazobactam was dose dependent: cerebrospinal fluid bacterial titers were reduced by 0.37 +/- 0.19 log10 CFU/ml per h with the lowest dose versus 0.96 +/- 0.25 log10 CFU/ml per h with the highest dose (P less than 0.001). At the relatively high doses of 160/20 and 200/25 mg of piperacillin-tazobactam per kg per h, the bactericidal activity of the combination was comparable to that of 10 and 25 mg of ceftriaxone per kg per h, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam D., de Visser I., Koeppe P. Pharmacokinetics of amoxicillin and clavulanic acid administered alone and in combination. Antimicrob Agents Chemother. 1982 Sep;22(3):353–357. doi: 10.1128/aac.22.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronoff S. C., Jacobs M. R., Johenning S., Yamabe S. Comparative activities of the beta-lactamase inhibitors YTR 830, sodium clavulanate, and sulbactam combined with amoxicillin or ampicillin. Antimicrob Agents Chemother. 1984 Oct;26(4):580–582. doi: 10.1128/aac.26.4.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken J. S., Bruun J. N., Gaustad P., Tasker T. C. Penetration of amoxicillin and potassium clavulanate into the cerebrospinal fluid of patients with inflamed meninges. Antimicrob Agents Chemother. 1986 Sep;30(3):481–484. doi: 10.1128/aac.30.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks G. F., Darrow W. W., Day J. A. Repeated gonorrhea: an analysis of importance and risk factors. J Infect Dis. 1978 Feb;137(2):161–169. doi: 10.1093/infdis/137.2.161. [DOI] [PubMed] [Google Scholar]
- Dacey R. G., Sande M. A. Effect of probenecid on cerebrospinal fluid concentrations of penicillin and cephalosporin derivatives. Antimicrob Agents Chemother. 1974 Oct;6(4):437–441. doi: 10.1128/aac.6.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decazes J. M., Bure A., Wolff M., Kitzis M. D., Pangon B., Modai J. Bactericidal activity against Haemophilus influenzae of cerebrospinal fluid of patients given amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1987 Dec;31(12):2018–2019. doi: 10.1128/aac.31.12.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decazes J. M., Ernst J. D., Sande M. A. Correlation of in vitro time-kill curves and kinetics of bacterial killing in cerebrospinal fluid during ceftriaxone therapy of experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1983 Oct;24(4):463–467. doi: 10.1128/aac.24.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio M., McCracken G. H., Jr, Nelson J. D., Chrane D., Shelton S. Pharmacokinetics and cerebrospinal fluid bactericidal activity of ceftriaxone in the treatment of pediatric patients with bacterial meningitis. Antimicrob Agents Chemother. 1982 Oct;22(4):622–627. doi: 10.1128/aac.22.4.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson G. M., Droller D. G., Greenman R. L., Hoffman T. A. Clinical evaluation of piperacillin with observations on penetrability into cerebrospinal fluid. Antimicrob Agents Chemother. 1981 Oct;20(4):481–486. doi: 10.1128/aac.20.4.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds G., McBride T. J., Knirsch A. K., Rodriguez W. J., Khan W. N. Penetration of sulbactam and ampicillin into cerebrospinal fluid of infants and young children with meningitis. Antimicrob Agents Chemother. 1987 Nov;31(11):1703–1705. doi: 10.1128/aac.31.11.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutmann L., Kitzis M. D., Yamabe S., Acar J. F. Comparative evaluation of a new beta-lactamase inhibitor, YTR 830, combined with different beta-lactam antibiotics against bacteria harboring known beta-lactamases. Antimicrob Agents Chemother. 1986 May;29(5):955–957. doi: 10.1128/aac.29.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. R., Aronoff S. C., Johenning S., Shlaes D. M., Yamabe S. Comparative activities of the beta-lactamase inhibitors YTR 830, clavulanate, and sulbactam combined with ampicillin and broad-spectrum penicillins against defined beta-lactamase-producing aerobic gram-negative bacilli. Antimicrob Agents Chemother. 1986 Jun;29(6):980–985. doi: 10.1128/aac.29.6.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. R., Aronoff S. C., Johenning S., Yamabe S. Comparative activities of the beta-lactamase inhibitors YTR 830, clavulanate and sulbactam combined with extended-spectrum penicillins against ticarcillin-resistant Enterobacteriaceae and pseudomonads. J Antimicrob Chemother. 1986 Aug;18(2):177–184. doi: 10.1093/jac/18.2.177. [DOI] [PubMed] [Google Scholar]
- McCracken G. H., Jr, Nelson J. D., Grimm L. Pharmacokinetics and bacteriological efficacy of cefoperazone, ceftriaxone, and moxalactam in experimental Streptococcus pneumoniae and Haemophilus influenzae meningitis. Antimicrob Agents Chemother. 1982 Feb;21(2):262–267. doi: 10.1128/aac.21.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizen L., Woodnutt G., Kernutt I., Catherall E. J. Simulation of human serum pharmacokinetics of ticarcillin-clavulanic acid and ceftazidime in rabbits, and efficacy against experimental Klebsiella pneumoniae meningitis. Antimicrob Agents Chemother. 1989 May;33(5):693–699. doi: 10.1128/aac.33.5.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata Y., McCracken G. H., Jr, Thomas M. L., Olsen K. D. Pharmacokinetics and therapeutic efficacy of imipenem, ceftazidime, and ceftriaxone in experimental meningitis due to an ampicillin- and chloramphenicol-resistant strain of Haemophilus influenzae type b. Antimicrob Agents Chemother. 1984 Jan;25(1):29–32. doi: 10.1128/aac.25.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaad U. B., McCracken G. H., Jr, Loock C. A., Thomas M. L. Pharmacokinetics and bacteriologic efficacy of moxalactam, cefotaxime, cefoperazone, and rocephin in experimental bacterial meningitis. J Infect Dis. 1981 Feb;143(2):156–163. doi: 10.1093/infdis/143.2.156. [DOI] [PubMed] [Google Scholar]
- Scheld W. M., Brodeur J. P., Sande M. A., Alliegro G. M. Comparison of cefoperazone with penicillin, ampicillin, gentamicin, and chloramphenicol in the therapy of experimental meningitis. Antimicrob Agents Chemother. 1982 Oct;22(4):652–656. doi: 10.1128/aac.22.4.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheld W. M., Brown R. S., Jr, Sande M. A. Comparison of netilmicin with gentamicin in the therapy of experimental Escherichia coli meningitis. Antimicrob Agents Chemother. 1978 Jun;13(6):899–904. doi: 10.1128/aac.13.6.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausbaugh L. J., Mandaleris C. D., Sande M. A. Comparison of four aminoglycoside antibiotics in the therapy of experimental E. coli meningitis. J Lab Clin Med. 1977 Apr;89(4):692–701. [PubMed] [Google Scholar]
- Strausbaugh L. J., Sande M. A. Factors influencing the therapy of experimental Proteus mirabilis meningitis in rabbits. J Infect Dis. 1978 Mar;137(3):251–260. doi: 10.1093/infdis/137.3.251. [DOI] [PubMed] [Google Scholar]
- Swartz M. N. Bacterial meningitis: more involved than just the meninges. N Engl J Med. 1984 Oct 4;311(14):912–914. doi: 10.1056/NEJM198410043111409. [DOI] [PubMed] [Google Scholar]
- Syrogiannopoulos G. A., Al-Sabbagh A., Olsen K. D., McCracken G. H., Jr Pharmacokinetics and bacteriological efficacy of ticarcillin-clavulanic acid (timentin) in experimental Escherichia coli K-1 and Haemophilus influenzae type b meningitis. Antimicrob Agents Chemother. 1987 Sep;31(9):1296–1300. doi: 10.1128/aac.31.9.1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Täuber M. G., Doroshow C. A., Hackbarth C. J., Rusnak M. G., Drake T. A., Sande M. A. Antibacterial activity of beta-lactam antibiotics in experimental meningitis due to Streptococcus pneumoniae. J Infect Dis. 1984 Apr;149(4):568–574. doi: 10.1093/infdis/149.4.568. [DOI] [PubMed] [Google Scholar]
- Täuber M. G., Sande M. A. Landmark perspective: The impact of penicillin on the treatment of meningitis. JAMA. 1984 Apr 13;251(14):1877–1880. doi: 10.1001/jama.1984.03340380059026. [DOI] [PubMed] [Google Scholar]
- Täuber M. G., Shibl A. M., Hackbarth C. J., Larrick J. W., Sande M. A. Antibiotic therapy, endotoxin concentration in cerebrospinal fluid, and brain edema in experimental Escherichia coli meningitis in rabbits. J Infect Dis. 1987 Sep;156(3):456–462. doi: 10.1093/infdis/156.3.456. [DOI] [PubMed] [Google Scholar]
- Woodnutt G., Kernutt I., Mizen L. Pharmacokinetics and distribution of ticarcillin-clavulanic acid (Timentin) in experimental animals. Antimicrob Agents Chemother. 1987 Nov;31(11):1826–1830. doi: 10.1128/aac.31.11.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]


