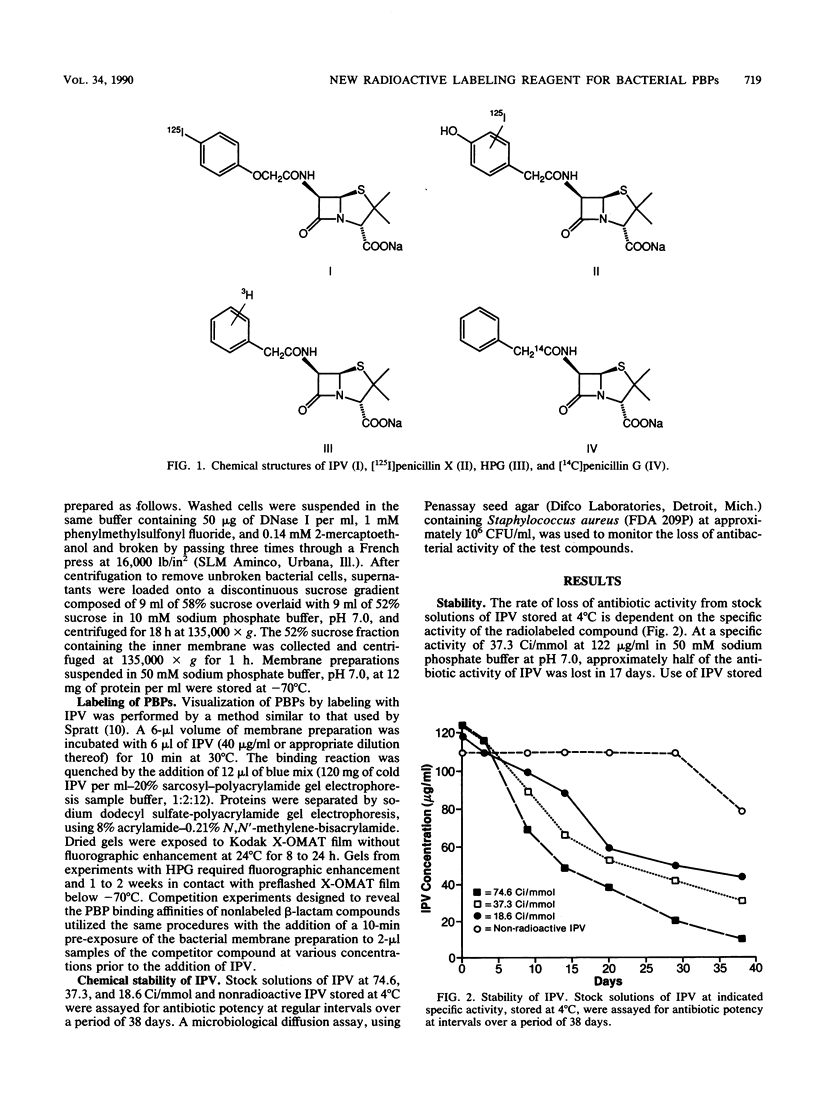
Abstract
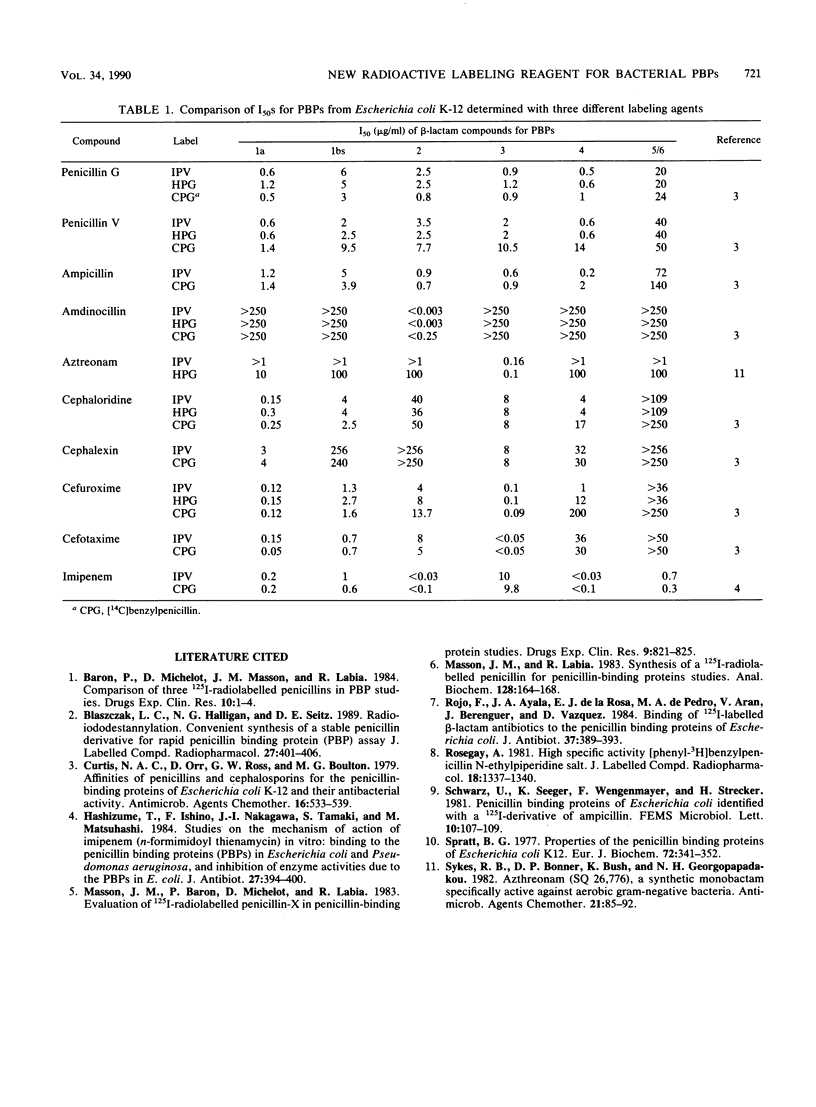
Radiolabeled penicillin G is widely used as the imaging agent in penicillin-binding protein (PBP) assays. The disadvantages of most forms of labeled penicillin G are instability on storage and the long exposure times usually required for autoradiography or fluorography of electrophoretic gels. We investigated the utility of radioiodinated penicillin V as an alternative reagent. Radioiodination of p-(trimethylstannyl)penicillin V with [125I]Na, using a modification of the chloramine-T method, is simple, high yielding, and site specific. We demonstrated the general equivalence of commercially obtained [3H]penicillin G and locally synthesized [125I]penicillin V (IPV) in their recognition of bacterial PBPs. Profiles of PBPs in membranes from Bacteroides fragilis, Escherichia coli, Providencia rettgeri, Staphylococcus aureus, Streptococcus pyogenes, Enterococcus faecalis, and Enterococcus faecium labeled with IPV or [3H]penicillin G were virtually identical. Use of IPV as the imaging agent in competition experiments for determination of the affinities of various beta-lactam antibiotics for the PBPs of E. coli yielded results similar to those obtained in experiments with [3H]penicillin G. Dried electrophoretic gels from typical PBP experiments, using IPV at 37.3 Ci/mmol and 30 micrograms/ml, exposed X-ray film in 8 to 24 h. The stability of IPV on storage at 4 degrees C was inversely proportional to specific activity. At 37.3 Ci/mmol and 60 micrograms/ml, IPV retained useful activity for at least 60 days at 4 degrees C. IPV represents a practical and stable reagent for rapid PBP assays.
Full text
PDF

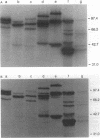
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis N. A., Orr D., Ross G. W., Boulton M. G. Affinities of penicillins and cephalosporins for the penicillin-binding proteins of Escherichia coli K-12 and their antibacterial activity. Antimicrob Agents Chemother. 1979 Nov;16(5):533–539. doi: 10.1128/aac.16.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume T., Ishino F., Nakagawa J., Tamaki S., Matsuhashi M. Studies on the mechanism of action of imipenem (N-formimidoylthienamycin) in vitro: binding to the penicillin-binding proteins (PBPs) in Escherichia coli and Pseudomonas aeruginosa, and inhibition of enzyme activities due to the PBPs in E. coli. J Antibiot (Tokyo) 1984 Apr;37(4):394–400. doi: 10.7164/antibiotics.37.394. [DOI] [PubMed] [Google Scholar]
- Masson J. M., Labia R. Synthesis of a 125I-radiolabeled penicillin for penicillin-binding proteins studies. Anal Biochem. 1983 Jan;128(1):164–168. doi: 10.1016/0003-2697(83)90357-3. [DOI] [PubMed] [Google Scholar]
- Rojo F., Ayala J. A., de la Rosa E. J., de Pedro M. A., Arán V., Berenguer J., Vázquez D. Binding of 125I-labeled beta-lactam antibiotics to the penicillin binding proteins of Escherichia coli. J Antibiot (Tokyo) 1984 Apr;37(4):389–393. doi: 10.7164/antibiotics.37.389. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]