Abstract
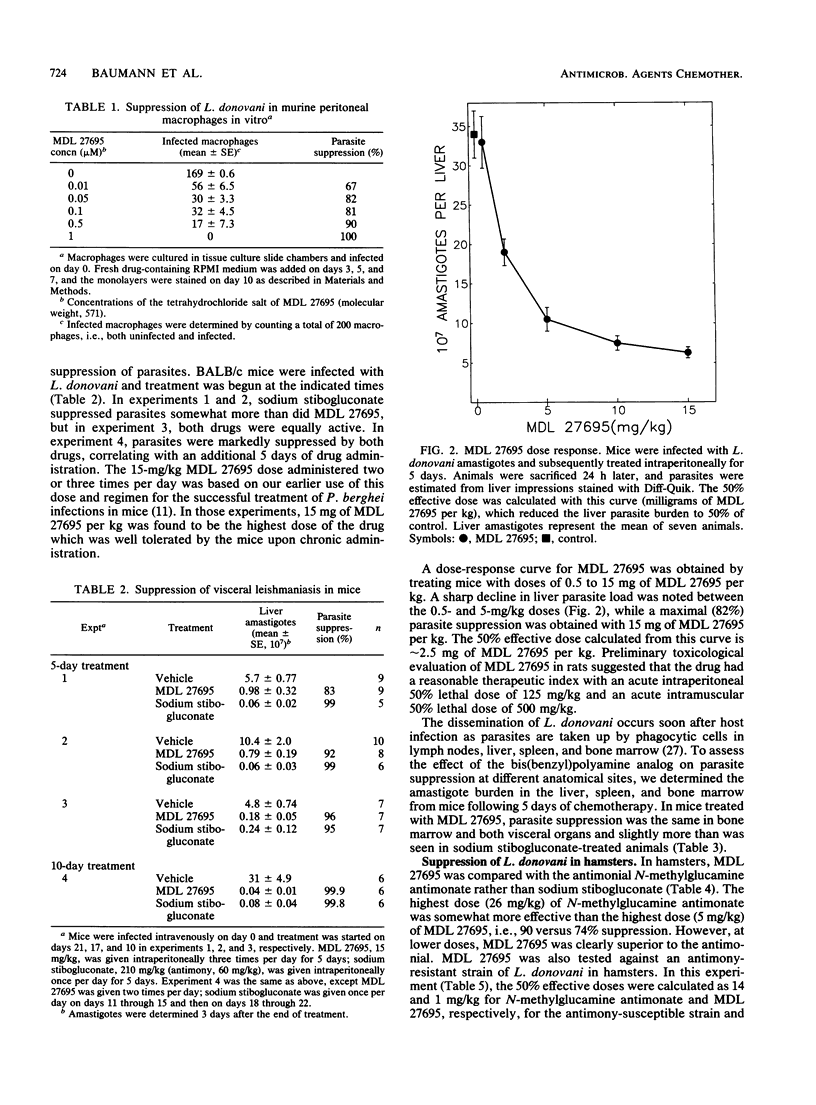
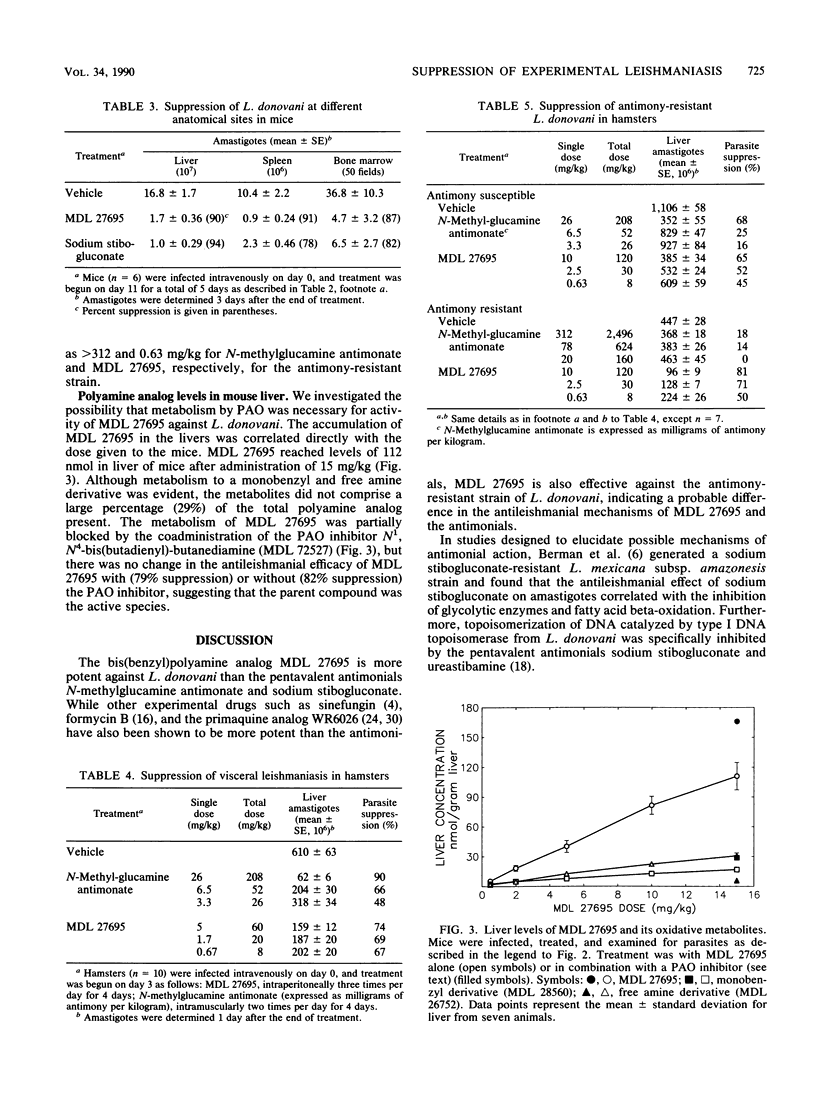
It was recently demonstrated that a bis(benzyl)polyamine analog (MDL 27695; N,N'-bis(3-[(phenylmethyl)amino]propyl)-1,7-diaminoheptane) possessed potent antimalarial activity in vitro and in vivo (A. J. Bitonti, J. A. Dumont, T. L. Bush, M. L. Edwards, D. M. Stemerick, P. P. McCann, and A. Sjoerdsma, Proc. Natl. Acad. Sci. USA 86:651-655, 1989). We now report that MDL 27695 also has potent antileishmanial activity, eliminating 77 to 100% of Leishmania donovani amastigotes from mouse peritoneal macrophages in vitro at 1 microM. Administration of 15 mg of MDL 27695 per kg three times per day for 5 days to L. donovani-infected mice suppressed parasite burdens in liver, spleen, and bone marrow by 83 to 96, 90, and 87%, respectively, and by 99.9% in livers of mice given the same dose two times per day for 10 days. Liver parasites were suppressed 74% in L. donovani-infected hamsters treated three times per day for 4 days with 5 mg of MDL 27695 per kg. The 50% effective doses for MDL 27695 were 2.5 mg/kg in mice and about 1 mg/kg in hamsters. In hamsters, MDL 27695 was equally effective against both antimony-susceptible and antimony-resistant L. donovani, suggesting a different mechanism of action for the two types of drugs. Coadministration of N1,N4-bis(butadienyl)-butanediamine (MDL 72527) to mice to inhibit host polyamine oxidase, and hence the formation of oxidative metabolites of MDL 27695, did not affect the antileishmanial activity of MDL 27695. Thus, the mechanism of action of MDL 27695 does not appear to be related to its oxidation to toxic metabolites but may involve interference with DNA and RNA syntheses as found previously in Plasmodium falciparum (Bitonti et al., Proc. Natl. Acad. Sci. USA 86:651-655, 1989).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adinolfi L. E., Bonventre P. F., Vander Pas M., Eppstein D. A. Synergistic effect of glucantime and a liposome-encapsulated muramyl dipeptide analog in therapy of experimental visceral leishmaniasis. Infect Immun. 1985 May;48(2):409–416. doi: 10.1128/iai.48.2.409-416.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alving C. R., Steck E. A., Chapman W. L., Jr, Waits V. B., Hendricks L. D., Swartz G. M., Jr, Hanson W. L. Therapy of leishmaniasis: superior efficacies of liposome-encapsulated drugs. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2959–2963. doi: 10.1073/pnas.75.6.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrach U., Schnur L. F., El-On J., Greenblatt C. L., Pearlman E., Robert-Gero M., Lederer E. Inhibitory activity of sinefungin and SIBA (5'-deoxy-5'-S-isobutylthio-adenosine) on the growth of promastigotes and amastigotes of different species of Leishmania. FEBS Lett. 1980 Dec 1;121(2):287–291. doi: 10.1016/0014-5793(80)80364-4. [DOI] [PubMed] [Google Scholar]
- Berman J. D. Chemotherapy for leishmaniasis: biochemical mechanisms, clinical efficacy, and future strategies. Rev Infect Dis. 1988 May-Jun;10(3):560–586. doi: 10.1093/clinids/10.3.560. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Edwards N., King M., Grogl M. Biochemistry of Pentostam resistant Leishmania. Am J Trop Med Hyg. 1989 Feb;40(2):159–164. doi: 10.4269/ajtmh.1989.40.159. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Goad L. J., Beach D. H., Holz G. G., Jr Effects of ketoconazole on sterol biosynthesis by Leishmania mexicana mexicana amastigotes in murine macrophage tumor cells. Mol Biochem Parasitol. 1986 Jul;20(1):85–92. doi: 10.1016/0166-6851(86)90145-3. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Hanson W. L., Chapman W. L., Alving C. R., Lopez-Berestein G. Antileishmanial activity of liposome-encapsulated amphotericin B in hamsters and monkeys. Antimicrob Agents Chemother. 1986 Dec;30(6):847–851. doi: 10.1128/aac.30.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. D., Lee L. S. Activity of antileishmanial agents against amastigotes in human monocyte-derived macrophages and in mouse peritoneal macrophages. J Parasitol. 1984 Apr;70(2):220–225. [PubMed] [Google Scholar]
- Bitonti A. J., Bush T. L., McCann P. P. Regulation of polyamine biosynthesis in rat hepatoma (HTC) cells by a bisbenzyl polyamine analogue. Biochem J. 1989 Feb 1;257(3):769–774. doi: 10.1042/bj2570769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., Dumont J. A., Bush T. L., Edwards M. L., Stemerick D. M., McCann P. P., Sjoerdsma A. Bis(benzyl)polyamine analogs inhibit the growth of chloroquine-resistant human malaria parasites (Plasmodium falciparum) in vitro and in combination with alpha-difluoromethylornithine cure murine malaria. Proc Natl Acad Sci U S A. 1989 Jan;86(2):651–655. doi: 10.1073/pnas.86.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitonti A. J., Dumont J. A., Bush T. L., Stemerick D. M., Edwards M. L., McCann P. P. Bis(benzyl)polyamine analogs as novel substrates for polyamine oxidase. J Biol Chem. 1990 Jan 5;265(1):382–388. [PubMed] [Google Scholar]
- Bitonti A. J., McCann P. P., Sjoerdsma A. The effects of polyamine analogues on malaria parasites in vitro and in vivo. Adv Exp Med Biol. 1988;250:717–726. doi: 10.1007/978-1-4684-5637-0_63. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D., Chulay J. D., Mugambi M., Were J. B., Gachihi G., Chunge C. N., Muigai R., Bhatt S. M., Ho M., Spencer H. C. Visceral leishmaniasis unresponsive to antimonial drugs. II. Response to high dosage sodium stibogluconate or prolonged treatment with pentamidine. Trans R Soc Trop Med Hyg. 1985;79(5):705–714. doi: 10.1016/0035-9203(85)90199-3. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Chang K. P. Phosphorylation and anti-leishmanial activity of formycin B. Biochem Biophys Res Commun. 1981 Jun 16;100(3):1377–1383. doi: 10.1016/0006-291x(81)91976-8. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Baillie A. J., Alexander J., Dolan T. F. The therapeutic effect of sodium stibogluconate in BALB/c mice infected with Leishmania donovani is organ-dependent. J Pharm Pharmacol. 1988 May;40(5):370–373. doi: 10.1111/j.2042-7158.1988.tb05271.x. [DOI] [PubMed] [Google Scholar]
- Chakraborty A. K., Majumder H. K. Mode of action of pentavalent antimonials: specific inhibition of type I DNA topoisomerase of Leishmania donovani. Biochem Biophys Res Commun. 1988 Apr 29;152(2):605–611. doi: 10.1016/s0006-291x(88)80081-0. [DOI] [PubMed] [Google Scholar]
- Croft S. L. Recent developments in the chemotherapy of leishmaniasis. Trends Pharmacol Sci. 1988 Oct;9(10):376–381. doi: 10.1016/0165-6147(88)90258-1. [DOI] [PubMed] [Google Scholar]
- Haidaris C. G., Bonventre P. F. Efficacy of combined immunostimulation and chemotherapy in experimental visceral Leishmaniasis. Am J Trop Med Hyg. 1983 Mar;32(2):286–295. doi: 10.4269/ajtmh.1983.32.286. [DOI] [PubMed] [Google Scholar]
- Hanson W. L., Chapman W. L., Jr, Kinnamon K. E. Testing of drugs for antileishmanial activity in golden hamsters infected with Leishmania donovani. Int J Parasitol. 1977 Dec;7(6):443–447. doi: 10.1016/0020-7519(77)90004-2. [DOI] [PubMed] [Google Scholar]
- Kaur K., Emmett K., McCann P. P., Sjoerdsma A., Ullman B. Effects of DL-alpha-difluoromethylornithine on Leishmania donovani promastigotes. J Protozool. 1986 Nov;33(4):518–521. doi: 10.1111/j.1550-7408.1986.tb05654.x. [DOI] [PubMed] [Google Scholar]
- Kinnamon K. E., Steck E. A., Loizeaux P. S., Hanson W. L., Chapman W. L., Jr, Waits V. B. The antileishmanial activity of lepidines. Am J Trop Med Hyg. 1978 Jul;27(4):751–757. doi: 10.4269/ajtmh.1978.27.751. [DOI] [PubMed] [Google Scholar]
- Liew F. Y. Functional heterogeneity of CD4+ T cells in leishmaniasis. Immunol Today. 1989 Feb;10(2):40–45. doi: 10.1016/0167-5699(89)90302-2. [DOI] [PubMed] [Google Scholar]
- Neal R. A., Croft S. L. An in-vitro system for determining the activity of compounds against the intracellular amastigote form of Leishmania donovani. J Antimicrob Chemother. 1984 Nov;14(5):463–475. doi: 10.1093/jac/14.5.463. [DOI] [PubMed] [Google Scholar]
- Neal R. A., Croft S. L., Nelson D. J. Anti-leishmanial effect of allopurinol ribonucleoside and the related compounds, allopurinol, thiopurinol, thiopurinol ribonucleoside, and of formycin B, sinefungin and the lepidine WR6026. Trans R Soc Trop Med Hyg. 1985;79(1):122–128. doi: 10.1016/0035-9203(85)90255-x. [DOI] [PubMed] [Google Scholar]
- Porter C. W., Sufrin J. R. Interference with polyamine biosynthesis and/or function by analogs of polyamines or methionine as a potential anticancer chemotherapeutic strategy. Anticancer Res. 1986 Jul-Aug;6(4):525–542. [PubMed] [Google Scholar]


