Abstract
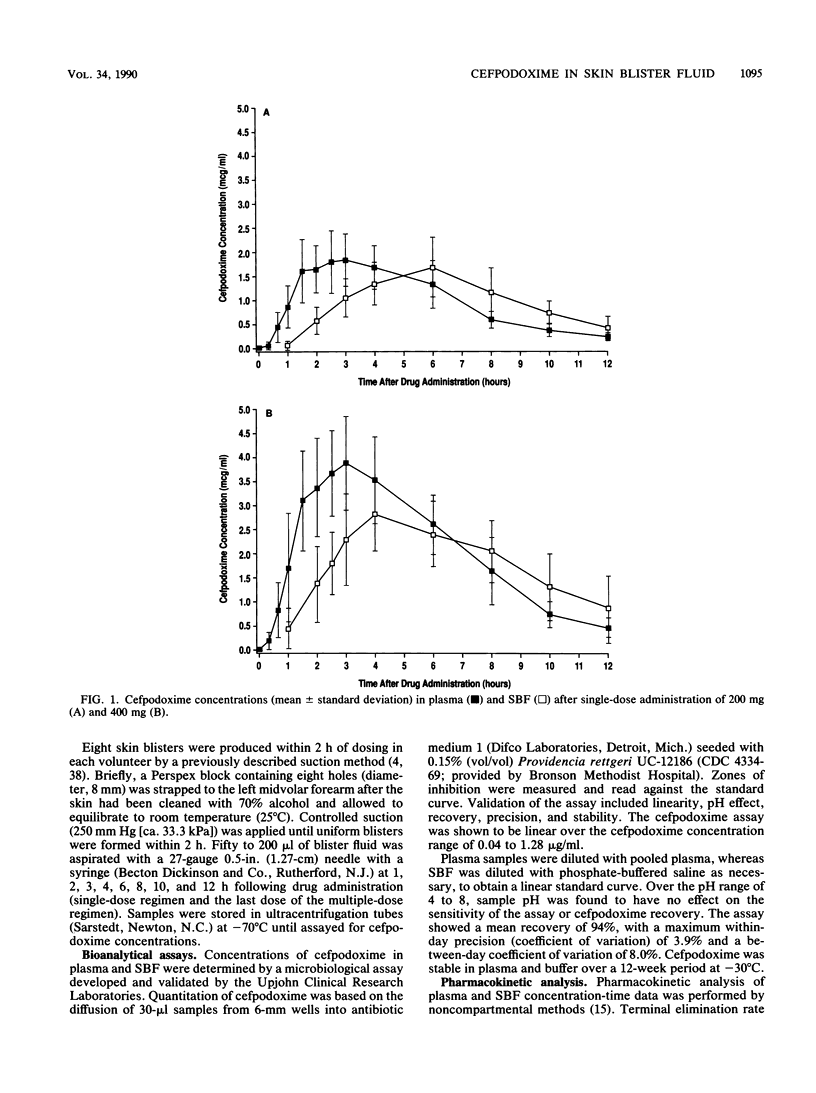
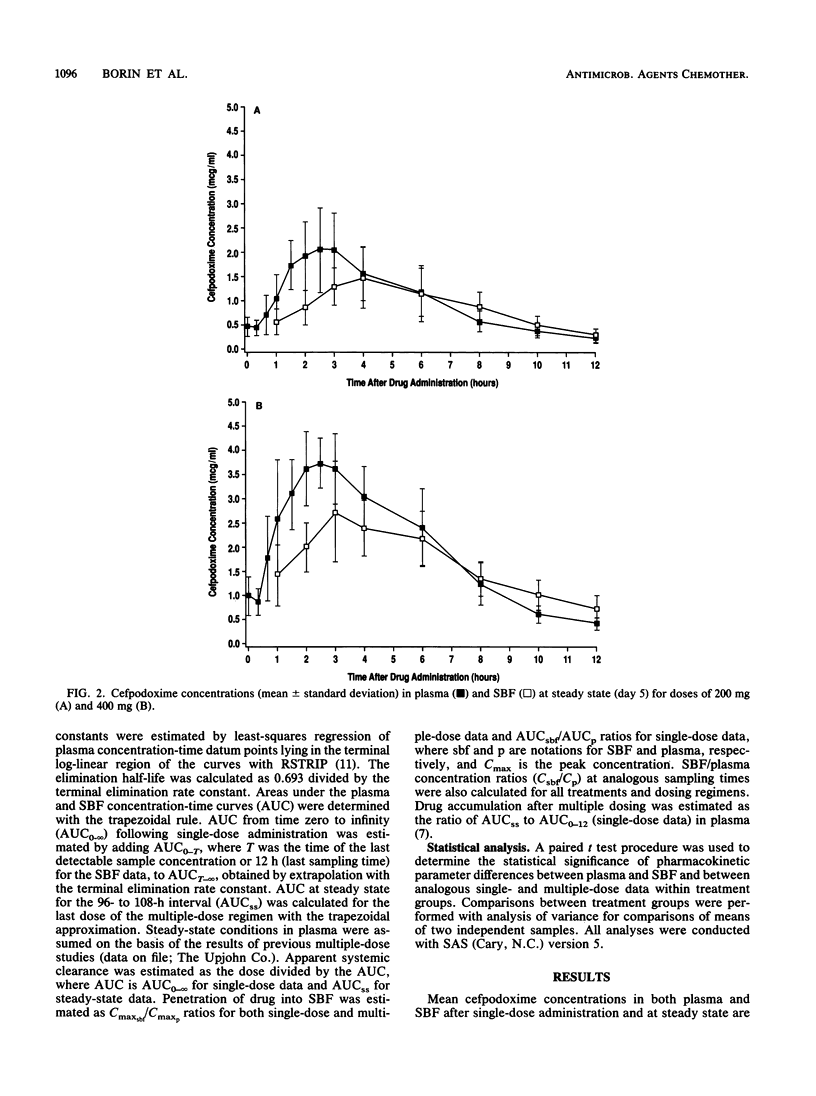
The single-dose and steady-state pharmacokinetics of cefpodoxime were assessed in plasma and skin blister fluid (SBF) after oral dosing of 200 mg (n = 8) and 400 mg (n = 8) of cefpodoxime proxetil (doses are expressed as cefpodoxime equivalents) in healthy subjects in an open-label, parallel-design study. Skin blisters were formed by air suction on the midvolar forearm by a previously validated method. After single-dose administration, serial plasma and SBF samples were collected over 24 h for measurement of cefpodoxime by microbiological assays. After a 1-week washout, subjects received the same doses of antibiotic every 12 h for 5 days, with plasma and SBF sampling on day 5. After 200 mg of cefpodoxime proxetil, average peak concentrations (Cmax) in plasma and SBF were 2.18 +/- 0.52 and 1.55 +/- 0.59 micrograms/ml, respectively, after a single dose and 2.33 +/- 0.74 and 1.56 +/- 0.55 micrograms/ml, respectively, at steady state. After 400 mg of cefpodoxime proxetil, Cmax in plasma and SBF averaged 4.16 +/- 1.04 and 2.94 +/- 0.71 micrograms/ml, respectively, following a single dose and 4.10 +/- 0.95 and 2.84 +/- 0.88 micrograms/ml, respectively, at steady state. Cmax occurred 1.1 to 1.6 h later in SBF than in plasma. There was no accumulation of cefpodoxime in plasma or SBF when dosing was done every 12 h. Cefpodoxime blister fluid penetration was estimated to be 67 to 101%, consistent with the relatively low serum protein binding of the drug. Cefpodoxime levels exceeding the MIC for 90% of many skin pathogens, such as Streptococcus species (<1 microgram/ml) or Staphylococcus species (2 to 4 micrograms/ml), were achieved in plasma and SBF following the 200- and/or 400-mg dosing regimens.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aoyama H., Izawa Y., Nishizaki A., Okuda J. Optimal conditions for injection of tobramycin and cefmenoxime into burn patients. Burns Incl Therm Inj. 1987 Aug;13(4):269–276. doi: 10.1016/0305-4179(87)90044-1. [DOI] [PubMed] [Google Scholar]
- Armstrong G. C., Wise R., Brown R. M., Hancox J. Comparison of ceftazidime and cefamandole pharmacokinetics and blister fluid concentrations. Antimicrob Agents Chemother. 1981 Sep;20(3):356–358. doi: 10.1128/aac.20.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan T. Pharmacokinetics of tissue penetration of antibiotics. Rev Infect Dis. 1981 Jan-Feb;3(1):45–66. doi: 10.1093/clinids/3.1.45. [DOI] [PubMed] [Google Scholar]
- Bergan T. Requirements for pharmacokinetic evaluation of antibiotics in phase I studies. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S319–S324. doi: 10.1093/clinids/8.supplement_3.s319. [DOI] [PubMed] [Google Scholar]
- Bernhardt L. L. Tissue and fluid concentrations of cefadroxil monohydrate. J Int Med Res. 1980;8(Suppl 1):58–63. [PubMed] [Google Scholar]
- Carbon C., Contrepois A., Beauvais C., Lamotte-Barrilon S. Enhanced interstitial ampicillin levels after oral bacampicillin. J Antimicrob Chemother. 1976 Sep;2(3):314–316. doi: 10.1093/jac/2.3.314. [DOI] [PubMed] [Google Scholar]
- Colburn W. A. Estimating the accumulation of drugs. J Pharm Sci. 1983 Jul;72(7):833–834. doi: 10.1002/jps.2600720734. [DOI] [PubMed] [Google Scholar]
- Craig W. A., Welling P. G. Protein binding of antimicrobials: clinical pharmacokinetic and therapeutic implications. Clin Pharmacokinet. 1977 Jul-Aug;2(4):252–268. doi: 10.2165/00003088-197702040-00002. [DOI] [PubMed] [Google Scholar]
- Cruciani M., Concia E., Passarella E., Soncini R. Penetration of cefonicid in skin suction blister fluid in healthy volunteers. Int J Clin Pharmacol Res. 1987;7(3):225–227. [PubMed] [Google Scholar]
- Fleishaker J. C., McNamara P. J. Performance of a diffusional clearance model for beta-lactam antimicrobial agents as influenced by extravascular protein binding and interstitial fluid kinetics. Antimicrob Agents Chemother. 1985 Sep;28(3):369–374. doi: 10.1128/aac.28.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frongillo R. F., Galuppo L., Moretti A. Comparative study on the interstitial passage of cefuroxime, cefoxitin, cefotaxime and cefoperazone in man by the suction skin blister method. Boll Ist Sieroter Milan. 1983 Nov 30;62(5):412–416. [PubMed] [Google Scholar]
- Frongillo R. F., Galuppo L., Moretti A. Suction skin blister, skin window, and skin chamber techniques to determine extravascular passage of cefotaxime in humans. Antimicrob Agents Chemother. 1981 Jan;19(1):22–28. doi: 10.1128/aac.19.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerding D. N., Hall W. H. The penetration of antibiotics into peritoneal fluid. Bull N Y Acad Med. 1975 Oct;51(9):1016–1019. [PMC free article] [PubMed] [Google Scholar]
- Holm S. E. Experimental models for studies on transportation of antibiotics to extravasal compartments. Scand J Infect Dis Suppl. 1978;(13):47–51. [PubMed] [Google Scholar]
- Howell A., Sutherland R., Rolinson G. N. Effect of protein binding on levels of ampicillin and cloxacillin in synovial fluid. Clin Pharmacol Ther. 1972 Sep-Oct;13(5):724–732. doi: 10.1002/cpt1972135part1724. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Barry A. L. Antimicrobial activity and disk diffusion susceptibility testing of U-76,253A (R-3746), the active metabolite of the new cephalosporin ester, U-76,252 (CS-807). Antimicrob Agents Chemother. 1988 Apr;32(4):443–449. doi: 10.1128/aac.32.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korting H. C. Plasma and skin blister fluid levels of cefotiam and cefmenoxime after single intramuscular application of 1 g in gonorrhea. Chemotherapy. 1984;30(5):277–282. doi: 10.1159/000238281. [DOI] [PubMed] [Google Scholar]
- Korting H. C., Schäfer-Korting M., Haag R., Mutschler E. Plasma, cantharides blister fluid, and suction blister fluid levels of ceftizoxime after single intramuscular application for gonorrhea. Int J Clin Pharmacol Ther Toxicol. 1984 Apr;22(4):218–220. [PubMed] [Google Scholar]
- LeBel M., Grégoire S., Caron M., Bergeron M. G. Difference in blister fluid penetration after single and multiple doses of ceftriaxone. Antimicrob Agents Chemother. 1985 Jul;28(1):123–127. doi: 10.1128/aac.28.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raeburn J. A. A review of experimental models for studying the tissue penetration of antibiotics in man. Scand J Infect Dis Suppl. 1978;(14):225–227. [PubMed] [Google Scholar]
- Reeves D. S., Bullock D. W., Bywater M. J., Holt H. A., White L. O., Thornhill D. P. The effect of probenecid on the pharmacokinetics and distribution of cefoxitin in healthy volunteers. Br J Clin Pharmacol. 1981 Apr;11(4):353–359. doi: 10.1111/j.1365-2125.1981.tb01132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner A., Hellum K. B., Digranes A., Bergman I. Transfer of penicillin G and ampicillin into human skin blisters induced by suction. Scand J Infect Dis Suppl. 1978;(14):233–237. [PubMed] [Google Scholar]
- Simon C., Malerczyk V., Klaus M. Absorption of bacampicillin and ampicillin and penetration into body fluids (skin blister fluid, saliva, tears) in healthy volunteers. Scand J Infect Dis Suppl. 1978;(14):228–232. [PubMed] [Google Scholar]
- Simon C., Malerczyk V., Tenschert B., Möhlenbeck F. Die geriatrische Pharmakologie von Cefazolin, Cefradin und Sulfisomidin. Arzneimittelforschung. 1976;26(7):1377–1382. [PubMed] [Google Scholar]
- Tan J. S., Salstrom S. J., File T. M. Diffusibility of the newer cephalosporins into human interstitial fluids. Am J Med. 1984 Oct 19;77(4C):33–36. [PubMed] [Google Scholar]
- Tan J. S., Trott A., Phair J. P., Watanakunakorn C. A method for measurement of antibiotics in human interstitial fluid. J Infect Dis. 1972 Nov;126(5):492–497. doi: 10.1093/infdis/126.5.492. [DOI] [PubMed] [Google Scholar]
- Utsui Y., Inoue M., Mitsuhashi S. In vitro and in vivo antibacterial activities of CS-807, a new oral cephalosporin. Antimicrob Agents Chemother. 1987 Jul;31(7):1085–1092. doi: 10.1128/aac.31.7.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstad R. A., Hellum K. B., Blika S., Dale L. G., Fredriksen T., Myhre K. I., Spencer G. R. Pharmacokinetics and tissue penetration of ceftazidime: studies on lymph, aqueous humour, skin blister, cerebrospinal and pleural fluid. J Antimicrob Chemother. 1983 Jul;12 (Suppl A):275–282. doi: 10.1093/jac/12.suppl_a.275. [DOI] [PubMed] [Google Scholar]
- Walstad R. A., Hellum K. B., Thurmann-Nielsen E., Dale L. G. Pharmacokinetics and tissue penetration of Timentin: a simultaneous study of serum, urine, lymph, suction blister and subcutaneous thread fluid. J Antimicrob Chemother. 1986 May;17 (Suppl 100):71–80. doi: 10.1093/jac/17.suppl_c.71. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M. A comparison of the pharmacokinetics and tissue penetration of ceftriaxone, moxalactam and cefotaxime. Eur J Clin Microbiol. 1983 Oct;2(5):505–508. doi: 10.1007/BF02013917. [DOI] [PubMed] [Google Scholar]
- Wise R., Bennett S. A., Dent J. The pharmacokinetics of orally absorbed cefuroxime compared with amoxycillin/clavulanic acid. J Antimicrob Chemother. 1984 Jun;13(6):603–610. doi: 10.1093/jac/13.6.603. [DOI] [PubMed] [Google Scholar]
- Wise R., Dyas A., Hegarty A., Andrews J. M. Pharmacokinetics and tissue penetration of azthreonam. Antimicrob Agents Chemother. 1982 Dec;22(6):969–971. doi: 10.1128/aac.22.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R., Gillett A. P., Cadge B., Durham S. R., Baker S. The influence of protein binding upon tissue fluid levels of six beta-lactam antibiotics. J Infect Dis. 1980 Jul;142(1):77–82. doi: 10.1093/infdis/142.1.77. [DOI] [PubMed] [Google Scholar]
- Wise R. Methods for evaluating the penetration of beta-lactam antibiotics into tissues. Rev Infect Dis. 1986 Jul-Aug;8 (Suppl 3):S325–S332. doi: 10.1093/clinids/8.supplement_3.s325. [DOI] [PubMed] [Google Scholar]


