Abstract
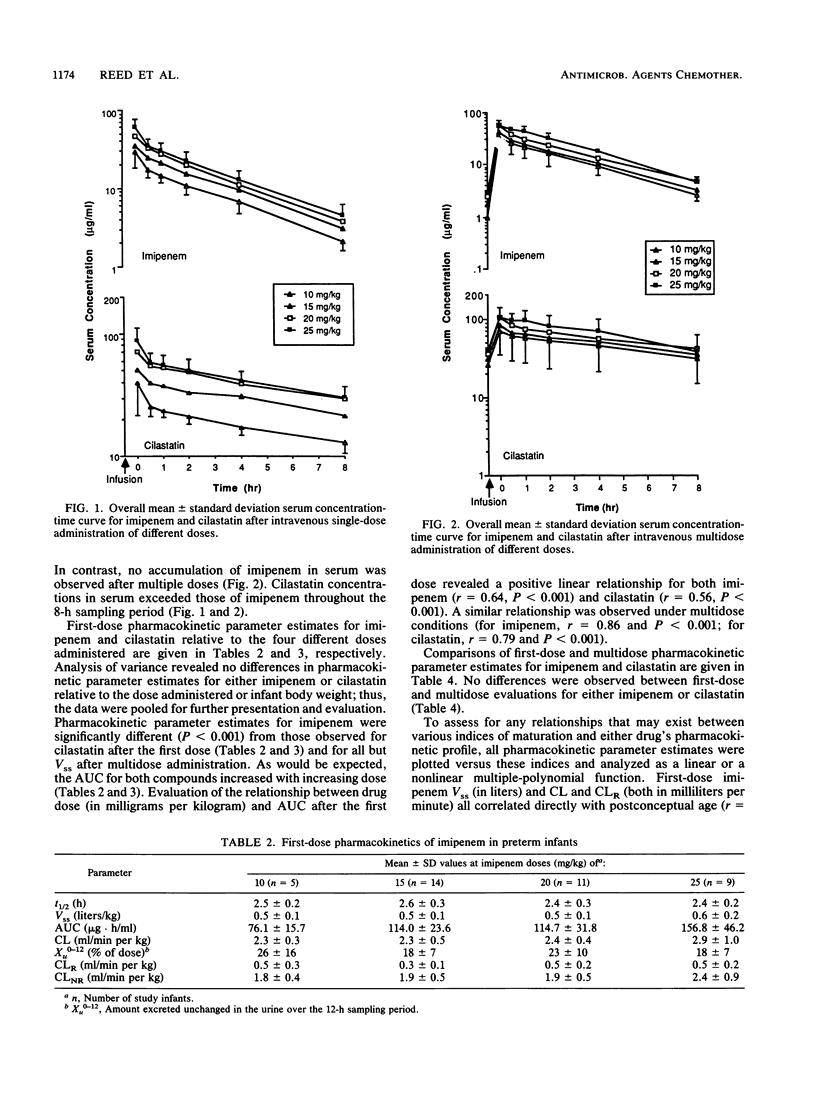
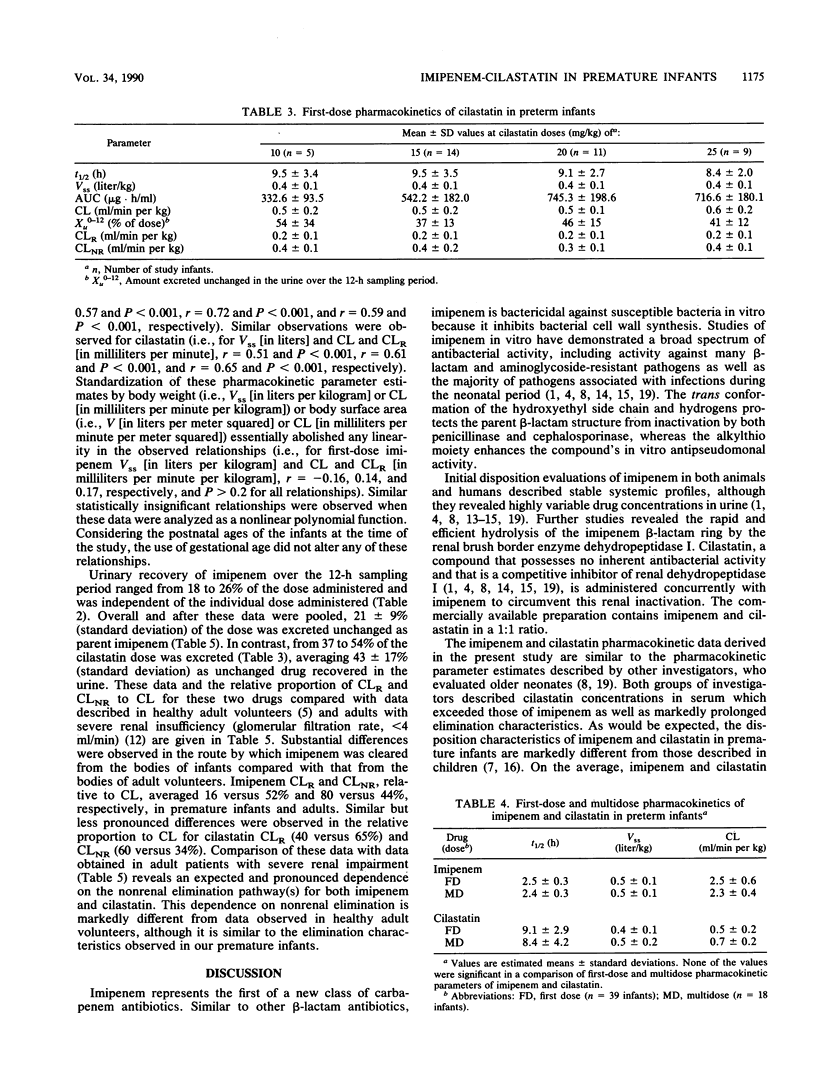
The first-dose and multidose pharmacokinetics of imipenem and cilastatin were evaluated in 41 premature infants during their first week of life. Premature infants (gestational age, less than or equal to 37 weeks) were assigned to receive 10-, 15-, 20-, or 25-mg/kg doses of imipenem-cilastatin (1:1) as a single- or multiple-dose regimen. A total of 39 infants received a single dose, whereas 18 infants received multiple doses. No differences were observed in pharmacokinetic parameter estimates for either agent relative to the dose administered or infant body weight; thus, the data were pooled. Elimination half-life, steady-state volume of distribution, and body clearance averaged 2.5 h, 0.5 liter/kg, and 2.5 ml/min per kg, respectively, for imipenem and 9.1 h, 0.4 liter/kg, and 0.5 ml/min per kg, respectively, for cilastatin. Similar values for these parameter estimates were observed after multidose administration, although substantial accumulation of cilastatin in serum was observed. A total of 21% of the imipenem and 43% of the cilastatin were excreted unchanged in the urine over a 12-h collection period. Corresponding renal clearances averaged 0.4 and 0.2 ml/min per kg for imipenem and cilastatin, respectively. Substantial differences were observed in the route by which imipenem was cleared from the body compared with data from adult volunteers. These data suggest that infants should receive an imipenem dose of 20 mg/kg administered every 12 h for the treatment of bacterial infections outside the central nervous system.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert G., Dagan R., Connor E., Campos J. M., Bloh A. M., Powell K. R., Plotkin S. A. Imipenem/cilastatin for the treatment of infections in hospitalized children. Am J Dis Child. 1985 Nov;139(11):1153–1156. doi: 10.1001/archpedi.1985.02140130091038. [DOI] [PubMed] [Google Scholar]
- Arant B. S., Jr Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr. 1978 May;92(5):705–712. doi: 10.1016/s0022-3476(78)80133-4. [DOI] [PubMed] [Google Scholar]
- Bauer L. A., Gibaldi M. Computation of model-independent pharmacokinetic parameters during multiple dosing. J Pharm Sci. 1983 Aug;72(8):978–979. doi: 10.1002/jps.2600720843. [DOI] [PubMed] [Google Scholar]
- Clissold S. P., Todd P. A., Campoli-Richards D. M. Imipenem/cilastatin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic efficacy. Drugs. 1987 Mar;33(3):183–241. doi: 10.2165/00003495-198733030-00001. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Standiford H. C., Bustamante C., Forrest A., Rivera G., Leslie J., Tatem B., Delaportas D., MacGregor R. R., Schimpff S. C. Multiple-dose pharmacokinetics of imipenem-cilastatin. Antimicrob Agents Chemother. 1984 Nov;26(5):715–721. doi: 10.1128/aac.26.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz L. M., Dubowitz V., Goldberg C. Clinical assessment of gestational age in the newborn infant. J Pediatr. 1970 Jul;77(1):1–10. doi: 10.1016/s0022-3476(70)80038-5. [DOI] [PubMed] [Google Scholar]
- Engelhard D., Shalit I., Stutman H. R., Greenwood R., Griffis J., Marks M. I. Single-dose pharmacokinetics of imipenem-cilastatin in pediatric patients. Pediatr Pharmacol (New York) 1986;5(4):273–279. [PubMed] [Google Scholar]
- Freij B. J., McCracken G. H., Jr, Olsen K. D., Threlkeld N. Pharmacokinetics of imipenem-cilastatin in neonates. Antimicrob Agents Chemother. 1985 Apr;27(4):431–435. doi: 10.1128/aac.27.4.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis-Hansen B. Water distribution in the foetus and newborn infant. Acta Paediatr Scand Suppl. 1983;305:7–11. doi: 10.1111/j.1651-2227.1983.tb09852.x. [DOI] [PubMed] [Google Scholar]
- Gibson T. P., Demetriades J. L., Bland J. A. Imipenem/cilastatin: pharmacokinetic profile in renal insufficiency. Am J Med. 1985 Jun 7;78(6A):54–61. doi: 10.1016/0002-9343(85)90102-0. [DOI] [PubMed] [Google Scholar]
- Gruber W. C., Rench M. A., Garcia-Prats J. A., Edwards M. S., Baker C. J. Single-dose pharmacokinetics of imipenem-cilastatin in neonates. Antimicrob Agents Chemother. 1985 Apr;27(4):511–514. doi: 10.1128/aac.27.4.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. F. Imipenem-cilastatin: the first thienamycin antibiotic. Pediatr Infect Dis. 1986 Jul-Aug;5(4):444–448. [PubMed] [Google Scholar]
- Jacobs R. F., Kearns G. L., Brown A. L., Longee D. C. Cerebrospinal fluid penetration of imipenem and cilastatin (primaxin) in children with central nervous system infections. Antimicrob Agents Chemother. 1986 Apr;29(4):670–674. doi: 10.1128/aac.29.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. F., Kearns G. L., Trang J. M., Brown A. L., Marmer B., McIntosh J. C., Underwood F. L., Kluza R. B. Single-dose pharmacokinetics of imipenem in children. J Pediatr. 1984 Dec;105(6):996–1001. doi: 10.1016/s0022-3476(84)80098-0. [DOI] [PubMed] [Google Scholar]
- Jarvis W. R. Epidemiology of nosocomial infections in pediatric patients. Pediatr Infect Dis J. 1987 Apr;6(4):344–351. doi: 10.1097/00006454-198704000-00003. [DOI] [PubMed] [Google Scholar]
- La Gamma E. F., Drusin L. M., Mackles A. W., Machalek S., Auld P. A. Neonatal infections. An important determinant of late NICU mortality in infants less than 1,000 g at birth. Am J Dis Child. 1983 Sep;137(9):838–841. doi: 10.1001/archpedi.1983.02140350016005. [DOI] [PubMed] [Google Scholar]
- Myers C. M., Blumer J. L. Determination of imipenem and cilastatin in serum by high-pressure liquid chromatography. Antimicrob Agents Chemother. 1984 Jul;26(1):78–81. doi: 10.1128/aac.26.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed M. D., Kliegman R. M., Weiner J. S., Huang M., Yamashita T. S., Blumer J. L. The clinical pharmacology of vancomycin in seriously ill preterm infants. Pediatr Res. 1987 Sep;22(3):360–363. doi: 10.1203/00006450-198709000-00024. [DOI] [PubMed] [Google Scholar]
- St Geme J. W., 3rd, Polin R. A. Neonatal sepsis. Progress in diagnosis and management. Drugs. 1988 Dec;36(6):784–800. doi: 10.2165/00003495-198836060-00007. [DOI] [PubMed] [Google Scholar]
- Swanson D. J., DeAngelis C., Smith I. L., Schentag J. J. Degradation kinetics of imipenem in normal saline and in human serum. Antimicrob Agents Chemother. 1986 May;29(5):936–937. doi: 10.1128/aac.29.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]


