Abstract
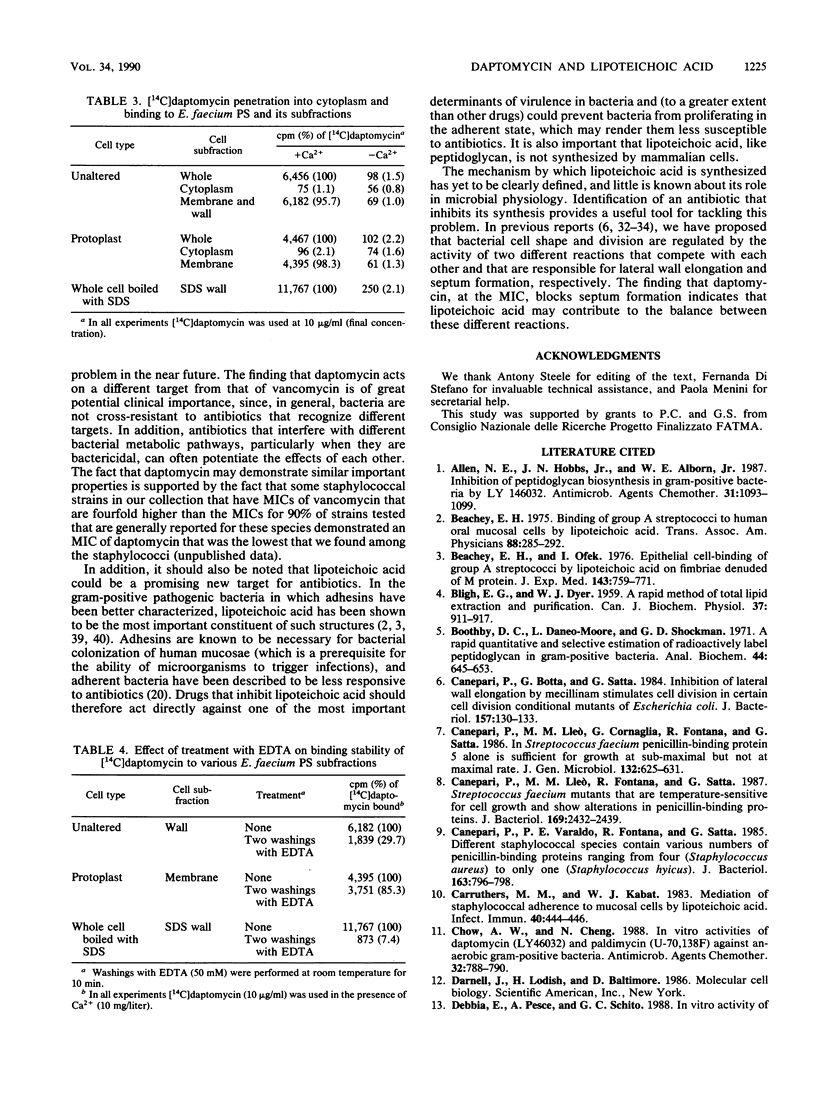
Daptomycin at the MIC allowed the cell mass increase of enterococcal strains and Bacillus subtilis to continue for 2 to 3 h at rates comparable to those of the controls. During this time the cell shape of the former changed to a rod configuration and that of the latter changed to long rods. In these bacteria, in which cell mass continued to increase, the MIC of daptomycin inhibited peptidoglycan synthesis by no more than 20% after 20 min of incubation and by roughly 50% after 2 h of incubation. Other macromolecules, such as DNA, RNA, and proteins, were only slightly affected. In contrast, incorporation of [14C]acetate into lipids was reduced by about 50% in the various strains after 20 min of treatment with daptomycin at the MIC. When the effect of the major lipid-containing polymers on synthesis was evaluated in detail, it was found that under conditions in which peptidoglycan and the other macromolecules mentioned above were inhibited only slightly (20%) and total lipid synthesis was inhibited by 50%, synthesis of teichoic and lipoteichoic acid was inhibited by 50 and 93%, respectively. Daptomycin was not found to enter the cytoplasm of either bacterial or mammalian cells. It bound, in the presence of calcium ions only, to whole bacterial cells, cell walls (both those that contained and those that did not contain membranes), and isolated membranes of bacterial and mammalian cells. Washing with EDTA removed daptomycin from all cells mentioned above and cell fractions except the bacterial membrane. It is concluded that lipoteichoic acid is most likely the primary target of daptomycin.
Full text
PDF

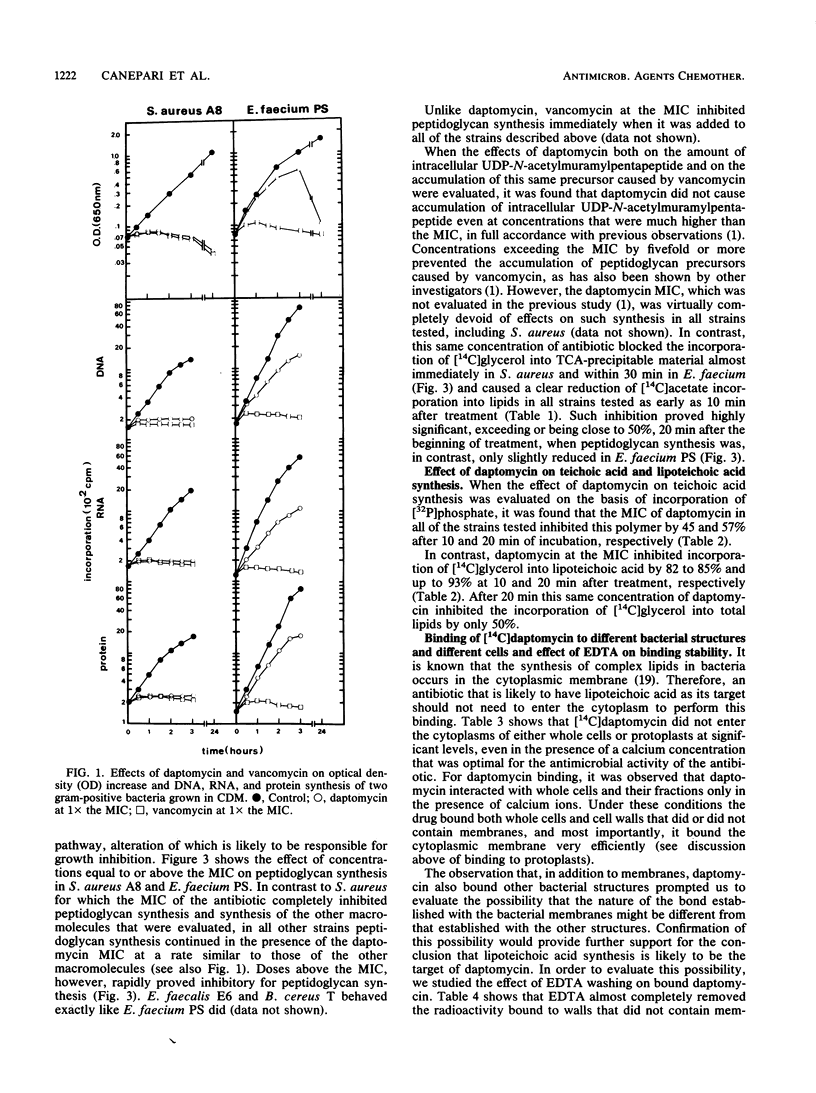

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen N. E., Hobbs J. N., Alborn W. E., Jr Inhibition of peptidoglycan biosynthesis in gram-positive bacteria by LY146032. Antimicrob Agents Chemother. 1987 Jul;31(7):1093–1099. doi: 10.1128/aac.31.7.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Binding of group A streptococci to human oral mucosal cells by lipoteichoic acid. Trans Assoc Am Physicians. 1975;88:285–292. [PubMed] [Google Scholar]
- Beachey E. H., Ofek I. Epithelial cell binding of group A streptococci by lipoteichoic acid on fimbriae denuded of M protein. J Exp Med. 1976 Apr 1;143(4):759–771. doi: 10.1084/jem.143.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boothby D., Daneo-Moore L., Shockman G. D. A rapid, guantitative, and selective estimation of radioactively labeled peptidoglycan in gram-positive bacteria. Anal Biochem. 1971 Dec;44(2):645–653. doi: 10.1016/0003-2697(71)90255-7. [DOI] [PubMed] [Google Scholar]
- Canepari P., Botta G., Satta G. Inhibition of lateral wall elongation by mecillinam stimulates cell division in certain cell division conditional mutants of Escherichia coli. J Bacteriol. 1984 Jan;157(1):130–133. doi: 10.1128/jb.157.1.130-133.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari P., Lleò M. M., Cornaglia G., Fontana R., Satta G. In Streptococcus faecium penicillin-binding protein 5 alone is sufficient for growth at sub-maximal but not at maximal rate. J Gen Microbiol. 1986 Mar;132(3):625–631. doi: 10.1099/00221287-132-3-625. [DOI] [PubMed] [Google Scholar]
- Canepari P., Lleò M. M., Fontana R., Satta G. Streptococcus faecium mutants that are temperature sensitive for cell growth and show alterations in penicillin-binding proteins. J Bacteriol. 1987 Jun;169(6):2432–2439. doi: 10.1128/jb.169.6.2432-2439.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canepari P., Varaldo P. E., Fontana R., Satta G. Different staphylococcal species contain various numbers of penicillin-binding proteins ranging from four (Staphylococcus aureus) to only one (Staphylococcus hyicus). J Bacteriol. 1985 Aug;163(2):796–798. doi: 10.1128/jb.163.2.796-798.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers M. M., Kabat W. J. Mediation of staphylococcal adherence to mucosal cells by lipoteichoic acid. Infect Immun. 1983 Apr;40(1):444–446. doi: 10.1128/iai.40.1.444-446.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow A. W., Cheng N. In vitro activities of daptomycin (LY146032) and paldimycin (U-70,138F) against anaerobic gram-positive bacteria. Antimicrob Agents Chemother. 1988 May;32(5):788–790. doi: 10.1128/aac.32.5.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Thauvin C., Gerson B., Moellering R. C., Jr In vitro activity and mechanism of action of A21978C1, a novel cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1985 Mar;27(3):357–362. doi: 10.1128/aac.27.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Willey S., Reiszner E., Spitzer P. G., Caputo G., Moellering R. C., Jr In vitro and in vivo activity of LY 146032, a new cyclic lipopeptide antibiotic. Antimicrob Agents Chemother. 1986 Oct;30(4):532–535. doi: 10.1128/aac.30.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elson L. A., Morgan W. T. A colorimetric method for the determination of glucosamine and chondrosamine. Biochem J. 1933;27(6):1824–1828. doi: 10.1042/bj0271824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faruki H., Niles A. C., Heeren R. L., Murray P. R. Effect of calcium on in vitro activity of LY146032 against Clostridium difficile. Antimicrob Agents Chemother. 1987 Mar;31(3):461–462. doi: 10.1128/aac.31.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- Gristina A. G., Jennings R. A., Naylor P. T., Myrvik Q. N., Webb L. X. Comparative in vitro antibiotic resistance of surface-colonizing coagulase-negative staphylococci. Antimicrob Agents Chemother. 1989 Jun;33(6):813–816. doi: 10.1128/aac.33.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovinen P., Kotilainen P. In vitro activity of a new cyclic lipopeptide antibiotic, LY146032, against gram-positive clinical bacteria. Antimicrob Agents Chemother. 1987 Mar;31(3):455–457. doi: 10.1128/aac.31.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Kaye D. Enterococci. Biologic and epidemiologic characteristics and in vitro susceptibility. Arch Intern Med. 1982 Oct 25;142(11):2006–2009. doi: 10.1001/archinte.142.11.2006. [DOI] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knapp C. C., Washington J. A., 2nd Antistaphylococcal activity of a cyclic peptide, LY146032, and vancomycin. Antimicrob Agents Chemother. 1986 Dec;30(6):938–939. doi: 10.1128/aac.30.6.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Pargwette A. R. Defective killing of enterococci: a common property of antimicrobial agents acting on the cell wall. Antimicrob Agents Chemother. 1980 Jun;17(6):965–968. doi: 10.1128/aac.17.6.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lleó M. M., Canepari P., Cornaglia G., Fontana R., Satta G. Bacteriostatic and bactericidal activities of beta-lactams against Streptococcus (Enterococcus) faecium are associated with saturation of different penicillin-binding proteins. Antimicrob Agents Chemother. 1987 Oct;31(10):1618–1626. doi: 10.1128/aac.31.10.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Watson B. K., Kunz L. J. Endocarditis due to group D streptococci. Comparison of disease caused by streptococcus bovis with that produced by the enterococci. Am J Med. 1974 Aug;57(2):239–250. doi: 10.1016/0002-9343(74)90448-3. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Wennersten C., Weinberg A. N. Studies on antibiotic synergism against enterococci. I. Bacteriologic studies. J Lab Clin Med. 1971 May;77(5):821–828. [PubMed] [Google Scholar]
- Roth G. S., Shockman G. D., Daneo-Moore L. Balanced macromolecular biosynthesis in "protoplasts" of Streptococcus faecalis. J Bacteriol. 1971 Mar;105(3):710–717. doi: 10.1128/jb.105.3.710-717.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Canepari P., Botta G., Fontana R. Control of cell septation by lateral wall extension in a pH-conditional morphology mutant of Klebsiella pneumoniae. J Bacteriol. 1980 Apr;142(1):43–51. doi: 10.1128/jb.142.1.43-51.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satta G., Fontana R., Canepari P., Botta G. Peptidoglycan synthesis in cocci and rods of a pH-dependent, morphologically conditional mutant of Klebsiella pneumoniae. J Bacteriol. 1979 Feb;137(2):727–734. doi: 10.1128/jb.137.2.727-734.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwalbe R. S., Stapleton J. T., Gilligan P. H. Emergence of vancomycin resistance in coagulase-negative staphylococci. N Engl J Med. 1987 Apr 9;316(15):927–931. doi: 10.1056/NEJM198704093161507. [DOI] [PubMed] [Google Scholar]
- Shockman G. D., Thompson J. S., Conover M. J. The autolytic enzyme system of Streptococcus faecalis. II. Partial characterization of the autolysin and its substrate. Biochemistry. 1967 Apr;6(4):1054–1065. doi: 10.1021/bi00856a014. [DOI] [PubMed] [Google Scholar]
- Storch G. A., Krogstad D. J. Antibiotic-induced lysis of enterococci. J Clin Invest. 1981 Sep;68(3):639–645. doi: 10.1172/JCI110298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti G., Chiofalo M. S., Tomasello F., Fava C., Mastroeni P. Mediation of Staphylococcus saprophyticus adherence to uroepithelial cells by lipoteichoic acid. Infect Immun. 1987 Mar;55(3):839–842. doi: 10.1128/iai.55.3.839-842.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti G., Tomasello F., Chiofalo M. S., Orefici G., Mastroeni P. Adherence of group B streptococci to adult and neonatal epithelial cells mediated by lipoteichoic acid. Infect Immun. 1987 Dec;55(12):3057–3064. doi: 10.1128/iai.55.12.3057-3064.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Baker C. N., Facklam R. R. Antibiotic susceptibility of Streptococcus bovis and other group D streptococci causing endocarditis. Antimicrob Agents Chemother. 1974 Mar;5(3):228–233. doi: 10.1128/aac.5.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbist L. In vitro activity of LY146032, a new lipopeptide antibiotic, against gram-positive cocci. Antimicrob Agents Chemother. 1987 Feb;31(2):340–342. doi: 10.1128/aac.31.2.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanger A. R., Murray B. E. Activity of LY146032 against Enterococci with and without high-level aminoglycoside resistance, including two penicillinase-producing strains. Antimicrob Agents Chemother. 1987 Nov;31(11):1779–1781. doi: 10.1128/aac.31.11.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W., Young F. E., Chatterjee A. N. Regulation of bacterial cell walls: turnover of cell wall in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):837–843. doi: 10.1128/jb.120.2.837-843.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]