Abstract
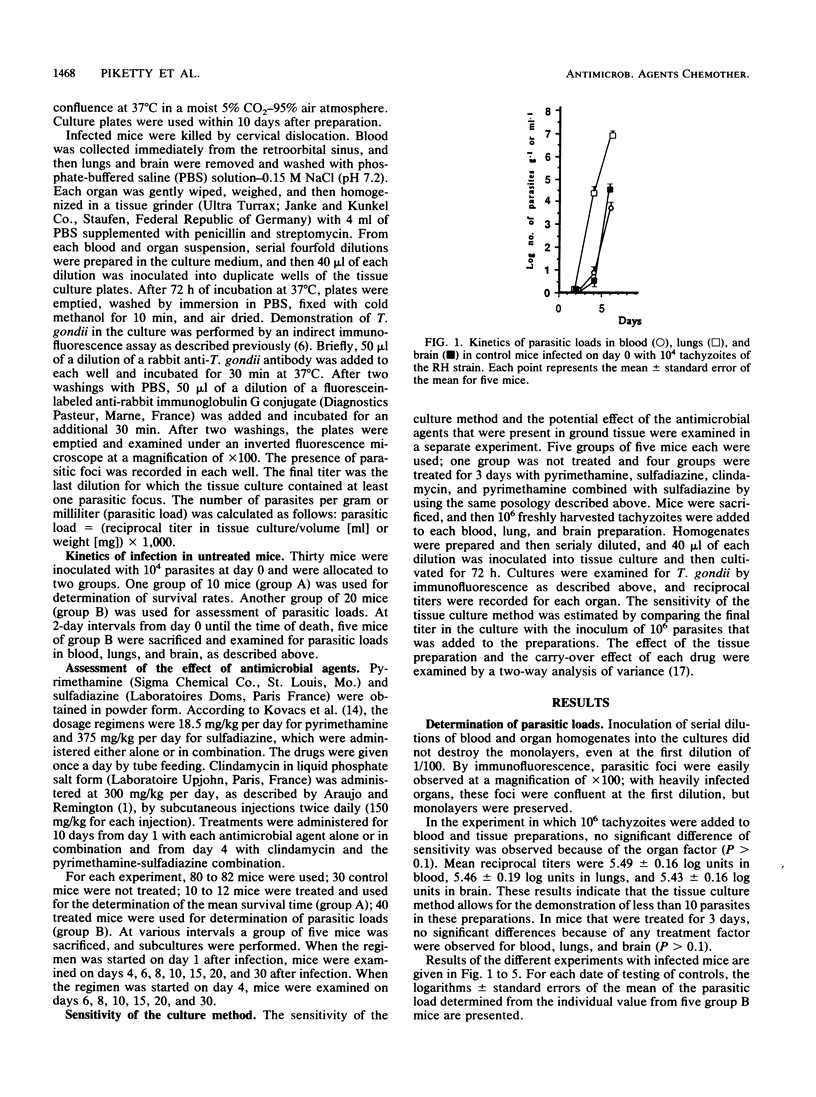
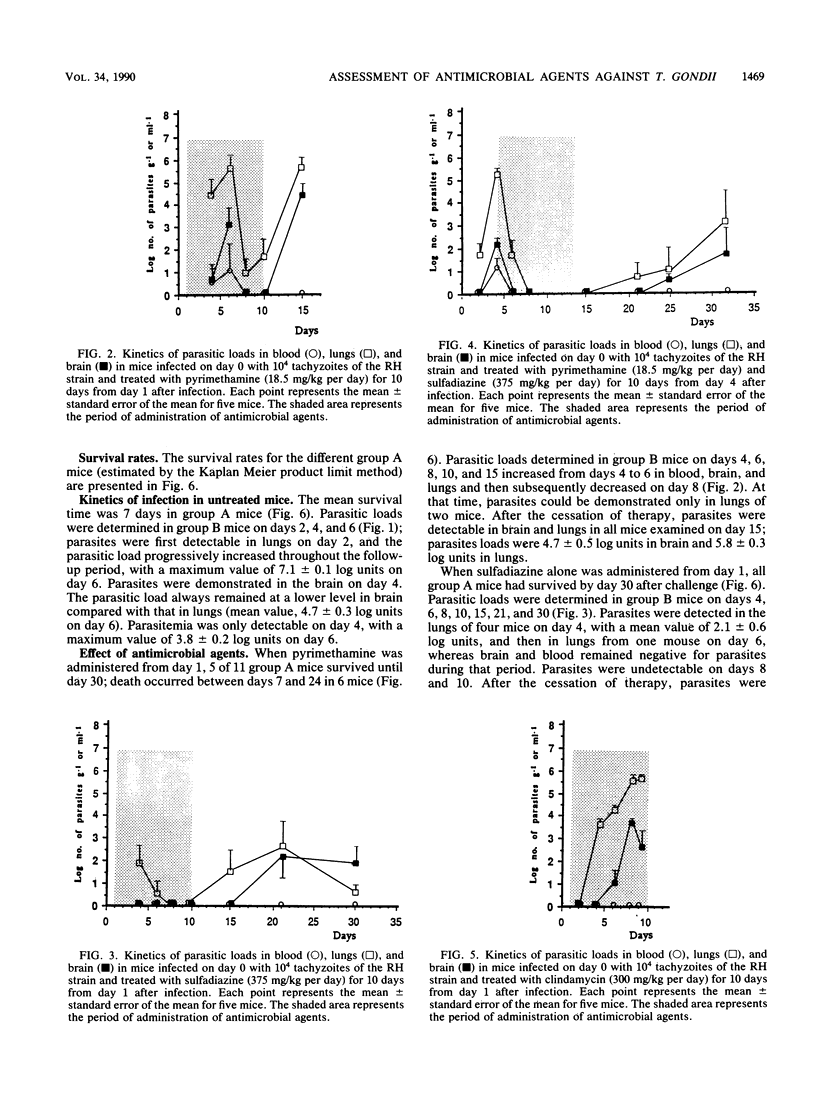
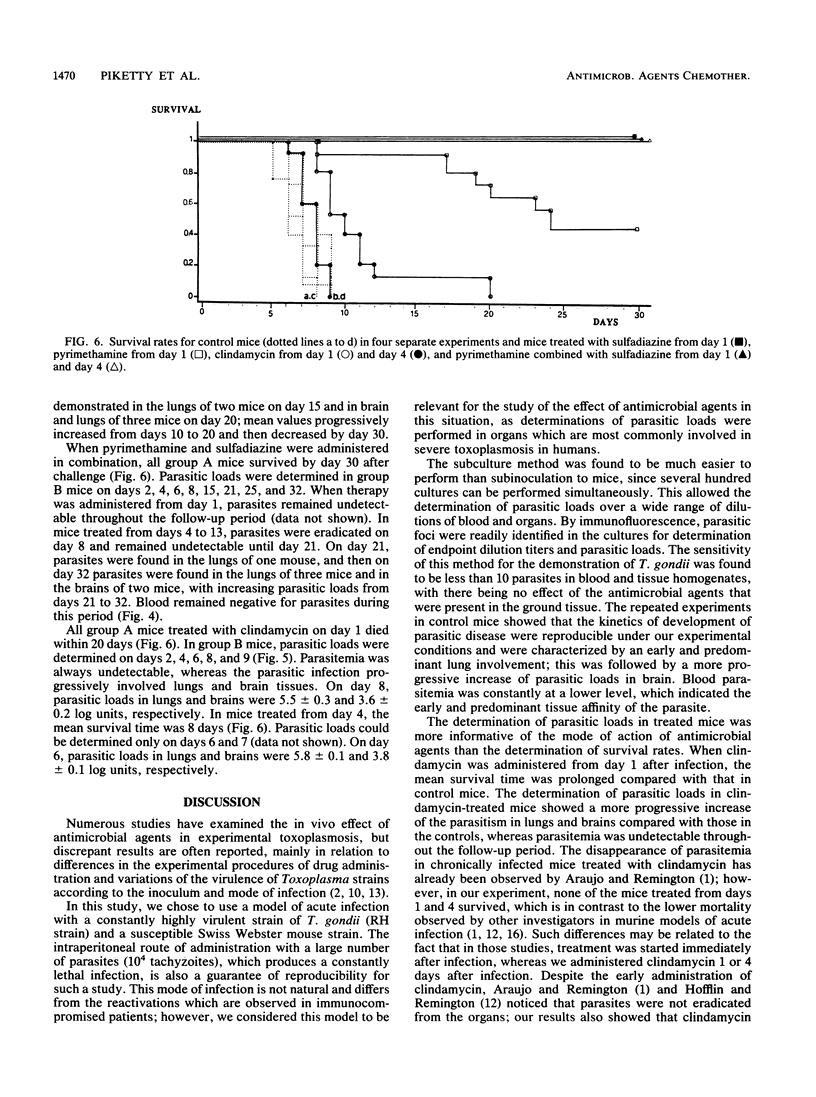
The in vivo effects of antimicrobial agents against Toxoplasma gondii were evaluated in mice that were infected intraperitoneally with 10(4) tachyzoites of the RH strain by determination of survival rates and study of the kinetics of growth of T. gondii in infected mice. At various intervals after infection, subcultures of serial dilutions of blood, lung, and brain homogenates were performed in fibroblast tissue cultures for determination of parasitic loads. Pyrimethamine (18.5 mg/kg per day), sulfadiazine (375 mg/kg per day), and clindamycin (300 mg/kg per day) were administered for 10 days from day 1 or day 4 after infection. Untreated control mice died within 9 days and showed early and predominant lung involvement. All mice treated with sulfadiazine administered from day 1 survived and were apparently healthy; parasitic loads decreased early after treatment, but a relapse was observed 5 days after the cessation of therapy. When pyrimethamine was administered from day 1, 7 of 11 mice died within 25 days; by determination of parasitic loads, the effect of pyrimethamine was only demonstrable from day 6, and a relapse was constantly observed after the cessation of therapy. When pyrimethamine and sulfadiazine were administered in combination, 100% of mice survived; when therapy was started at day 1, parasites remained undetectable; in mice treated from day 4, parasites were eradicated by day 8 but infection relapsed 8 days after the cessation of therapy. All mice treated with clindamycin from day 1 or day 4 died within 10 days, but parasitemia was always undetectable. These results indicate that study of the kinetics of parasitic loads in blood and organs may provide additional information on the effect of antimicrobial agents against T. gondii in regard to the evolution of the infection and may represent a reliable basis for the determination of therapeutic regimens in humans.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araujo F. G., Remington J. S. Effect of clindamycin on acute and chronic toxoplasmosis in mice. Antimicrob Agents Chemother. 1974 Jun;5(6):647–651. doi: 10.1128/aac.5.6.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo F. G., Williams D. M., Grumet F. C., Remington J. S. Strain-dependent differences in murine susceptibility to toxoplasma. Infect Immun. 1976 May;13(5):1528–1530. doi: 10.1128/iai.13.5.1528-1530.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhni E., Fust B., Rieder J., Schaerer K., Havas L. Comparative toxicological, chemotherapeutic and pharmacokinetic studies with sulphormethoxine and other sulphonamides in animals and man. Chemotherapy. 1969;14(4):195–226. doi: 10.1159/000220630. [DOI] [PubMed] [Google Scholar]
- Coleman M. D., Mihaly G. W., Edwards G., Ward S. A., Howells R. E., Breckenridge A. M. Pyrimethamine pharmacokinetics and its tissue localization in mice: effect of dose size. J Pharm Pharmacol. 1985 Mar;37(3):170–174. doi: 10.1111/j.2042-7158.1985.tb05034.x. [DOI] [PubMed] [Google Scholar]
- Derouin F., Chastang C. In vitro effects of folate inhibitors on Toxoplasma gondii. Antimicrob Agents Chemother. 1989 Oct;33(10):1753–1759. doi: 10.1128/aac.33.10.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Mazeron M. C., Garin Y. J. Comparative study of tissue culture and mouse inoculation methods for demonstration of Toxoplasma gondii. J Clin Microbiol. 1987 Sep;25(9):1597–1600. doi: 10.1128/jcm.25.9.1597-1600.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derouin F., Nalpas J., Chastang C. Mesure in vitro de l'effet inhibiteur de macrolides, lincosamides et synergestines sur la croissance de Toxoplasma gondii. Pathol Biol (Paris) 1988 Dec;36(10):1204–1210. [PubMed] [Google Scholar]
- EYLES D. E. The present status of the chemotherapy of toxoplasmosis. Am J Trop Med Hyg. 1953 May;2(3):429–444. doi: 10.4269/ajtmh.1953.2.429. [DOI] [PubMed] [Google Scholar]
- FRENKEL J. K. Host, strain and treatment variation as factors in the pathogenesis of toxoplasmosis. Am J Trop Med Hyg. 1953 May;2(3):390–415. doi: 10.4269/ajtmh.1953.2.390. [DOI] [PubMed] [Google Scholar]
- Hofflin J. M., Conley F. K., Remington J. S. Murine model of intracerebral toxoplasmosis. J Infect Dis. 1987 Mar;155(3):550–557. doi: 10.1093/infdis/155.3.550. [DOI] [PubMed] [Google Scholar]
- Hofflin J. M., Remington J. S. Clindamycin in a murine model of toxoplasmic encephalitis. Antimicrob Agents Chemother. 1987 Apr;31(4):492–496. doi: 10.1128/aac.31.4.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. M. Strain-dependent, route of challenge-dependent, murine susceptibility to toxoplasmosis. Z Parasitenkd. 1984;70(3):303–309. doi: 10.1007/BF00927816. [DOI] [PubMed] [Google Scholar]
- Kovacs J. A., Allegra C. J., Chabner B. A., Swan J. C., Drake J., Lunde M., Parrillo J. E., Masur H. Potent effect of trimetrexate, a lipid-soluble antifolate, on Toxoplasma gondii. J Infect Dis. 1987 May;155(5):1027–1032. doi: 10.1093/infdis/155.5.1027. [DOI] [PubMed] [Google Scholar]
- McMaster P. R., Powers K. G., Finerty J. F., Lunde M. N. The effect of two chlorinated lincomycin analogues against acute toxoplasmosis in mice. Am J Trop Med Hyg. 1973 Jan;22(1):14–17. doi: 10.4269/ajtmh.1973.22.14. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Conley F. K., Remington J. S. Importance of endogenous IFN-gamma for prevention of toxoplasmic encephalitis in mice. J Immunol. 1989 Sep 15;143(6):2045–2050. [PubMed] [Google Scholar]


