Abstract
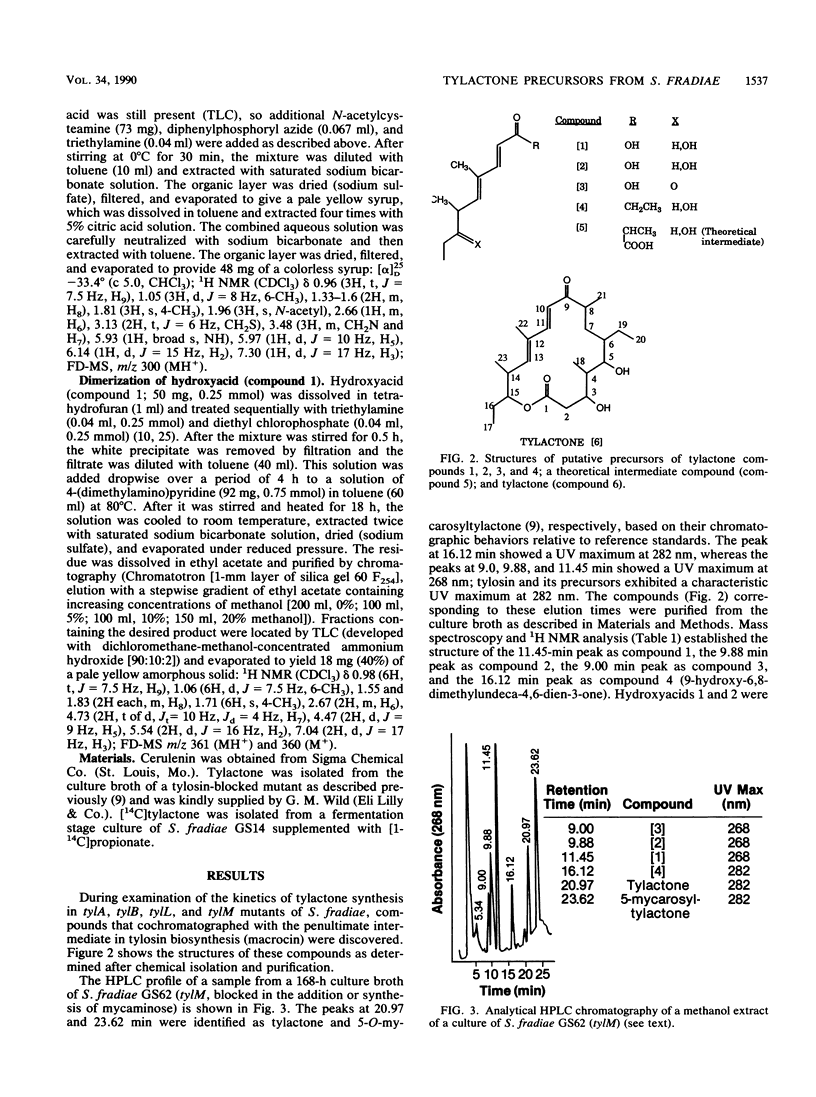
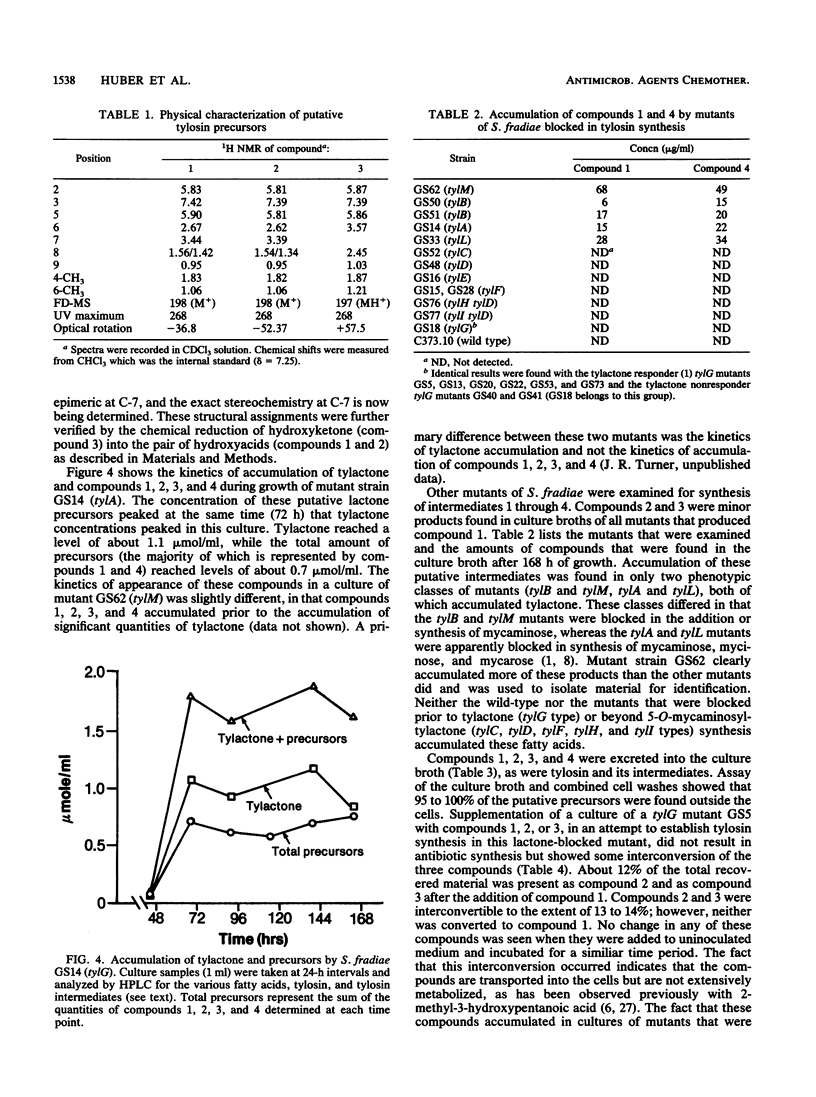
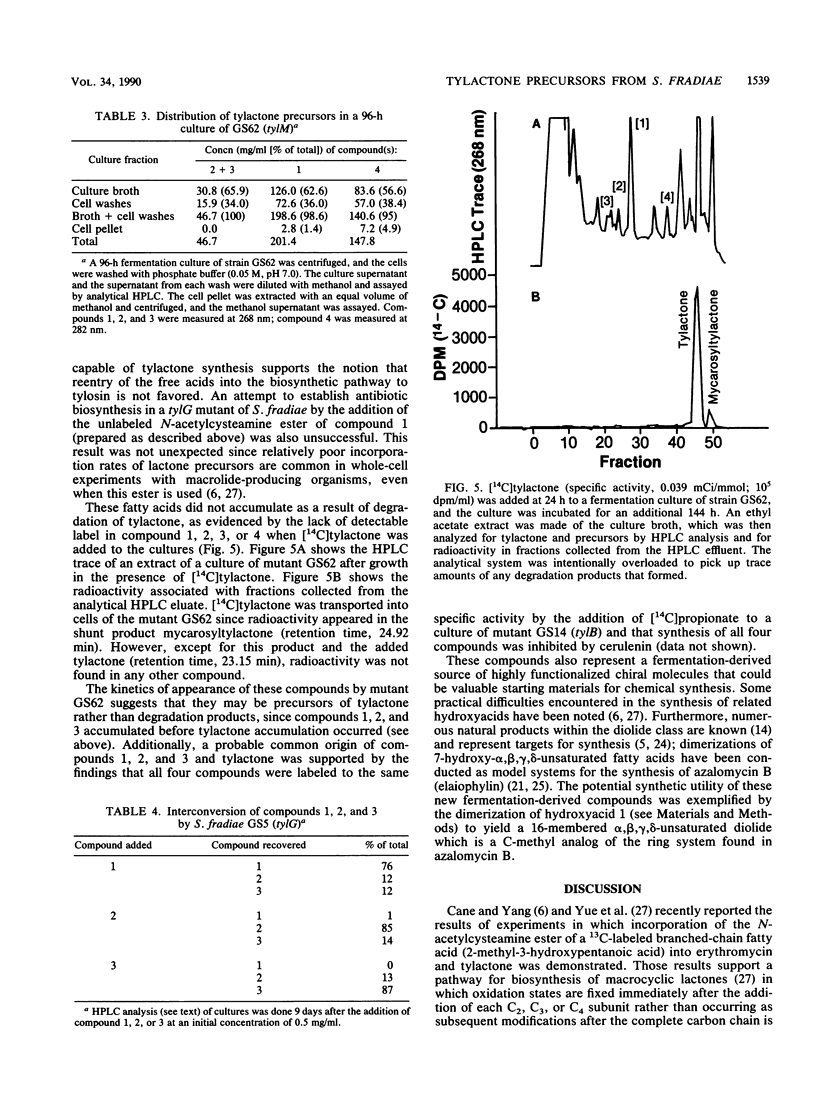
Three branched-chain fatty acids (7-hydroxy-4,6-dimethylnona-2,4-dienoic acid [compound 1], its 7-epimer [compound 2], and 7-keto-4,6-dimethylnona-2,4-dienoic acid [compound 3]) and a ketone (9-hydroxy-6,8-dimethylundeca-4,6-dien-3-one [compound 4]) were isolated from the culture broth of mutants of Streptomyces fradiae which were blocked in the biosynthesis of the macrolide antibiotic tylosin. Two phenotypic classes of mutants of this organism which were blocked in the addition of mycaminose to tylactone (compound 6) accumulated these compounds. These compounds were not produced by mutants which were blocked in lactone synthesis, in steps beyond mycaminose addition, or by the wild-type strain. Synthesis of these compounds, like synthesis of tylosin, was inhibited by the addition of cerulenin. Compounds 1, 2, and 3 were partially interconvertible by these mutants; but they were not produced from the degradation of tylactone and they were not directly incorporated into tylosin by intact cells. The structures of compounds 1 and 2 were equivalent to that of a predicted intermediate (S. Yue, J. S. Duncan, Y. Yamamoto, and C. R. Hutchinson, J. Am. Chem. Soc. 109:1253-1255, 1987) in the biosynthesis of tylactone. The ketone (compound 4) reported previously (N. D. Jones, M. O. Chaney, H. A. Kirst, G. M. Wild, R. H. Baltz, R. L. Hamill, and J. W. Paschal, J. Antibiot. 35:420-425, 1982) appears to be the decarboxylation product of the intermediate following that represented by compound 1. This represents the first report of the isolation of putative precursors of tylactone from tylosin-producing organisms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltz R. H., Seno E. T. Genetics of Streptomyces fradiae and tylosin biosynthesis. Annu Rev Microbiol. 1988;42:547–574. doi: 10.1146/annurev.mi.42.100188.002555. [DOI] [PubMed] [Google Scholar]
- Baltz R. H., Seno E. T. Properties of Streptomyces fradiae mutants blocked in biosynthesis of the macrolide antibiotic tylosin. Antimicrob Agents Chemother. 1981 Aug;20(2):214–225. doi: 10.1128/aac.20.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltz R. H., Seno E. T., Stonesifer J., Wild G. M. Biosynthesis of the macrolide antibiotic tylosin. A preferred pathway from tylactone to tylosin. J Antibiot (Tokyo) 1983 Feb;36(2):131–141. doi: 10.7164/antibiotics.36.131. [DOI] [PubMed] [Google Scholar]
- Cox K. L., Fishman S. E., Larson J. L., Stanzak R., Reynolds P. A., Yeh W. K., van Frank R. M., Birmingham V. A., Hershberger C. L., Seno E. T. The use of recombinant DNA techniques to study tylosin biosynthesis and resistance in Streptomyces fradiae. J Nat Prod. 1986 Nov-Dec;49(6):971–980. doi: 10.1021/np50048a002. [DOI] [PubMed] [Google Scholar]
- Fishman S. E., Cox K., Larson J. L., Reynolds P. A., Seno E. T., Yeh W. K., Van Frank R., Hershberger C. L. Cloning genes for the biosynthesis of a macrolide antibiotic. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8248–8252. doi: 10.1073/pnas.84.23.8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N. D., Chaney M. O., Kirst H. A., Wild G. M., Baltz R. H., Hamill R. L., Paschal J. W. Novel fermentation products from Streptomyces fradiae: X-ray crystal structure of 5-O-mycarosyltylactone and proof of the absolute configuration of tylosin. J Antibiot (Tokyo) 1982 Apr;35(4):420–425. doi: 10.7164/antibiotics.35.420. [DOI] [PubMed] [Google Scholar]
- KANEDA T., BUTTE J. C., TAUBMAN S. B., CORCORAN J. W. Actinomycete antibiotics. III. The biogenesis of erythronolide, the C-21 branched chain lactone in erythromycin. J Biol Chem. 1962 Feb;237:322–328. [PubMed] [Google Scholar]
- Omura S., Kitao C., Hamada H., Ikeda H. Bioconversion and biosynthesis of 16-membered macrolide antibiotics. X. Final steps in the biosynthesis of spiramycin, using enzyme inhibitor: cerulenin. Chem Pharm Bull (Tokyo) 1979 Jan;27(1):176–182. doi: 10.1248/cpb.27.176. [DOI] [PubMed] [Google Scholar]
- Omura S., Kitao C., Miyazawa J., Imai H., Takeshima H. Bioconversion and biosynthesis of 16-membered macrolide antibiotic, tylosin, using enzyme inhibitor: cerulenin. J Antibiot (Tokyo) 1978 Mar;31(3):254–256. doi: 10.7164/antibiotics.31.254. [DOI] [PubMed] [Google Scholar]
- Omura S., Nakagawa A., Neszmélyi A., Gero S. D., Sepulchre A. M., Piriou F., Lukacs G. Carbon-13 nuclear magnetic resonance spectral analysis of 16-membered macrolide antibiotics. J Am Chem Soc. 1975 Jul 9;97(14):4001–4009. doi: 10.1021/ja00847a022. [DOI] [PubMed] [Google Scholar]
- Omura S., Takeshima H. Inhibition of the biosynthesis of leucomycin, a macrolide antibiotic, by cerulenin. J Biochem. 1974 Jan;75(1):193–195. doi: 10.1093/oxfordjournals.jbchem.a130375. [DOI] [PubMed] [Google Scholar]
- Omura S., Takeshima H., Nakagawa A., Miyazawa J. The biosynthesis of picromycin using 13C enriched precursors. J Antibiot (Tokyo) 1976 Mar;29(3):316–317. doi: 10.7164/antibiotics.29.316. [DOI] [PubMed] [Google Scholar]
- Omura S. The antibiotic cerulenin, a novel tool for biochemistry as an inhibitor of fatty acid synthesis. Bacteriol Rev. 1976 Sep;40(3):681–697. doi: 10.1128/br.40.3.681-697.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


