Abstract
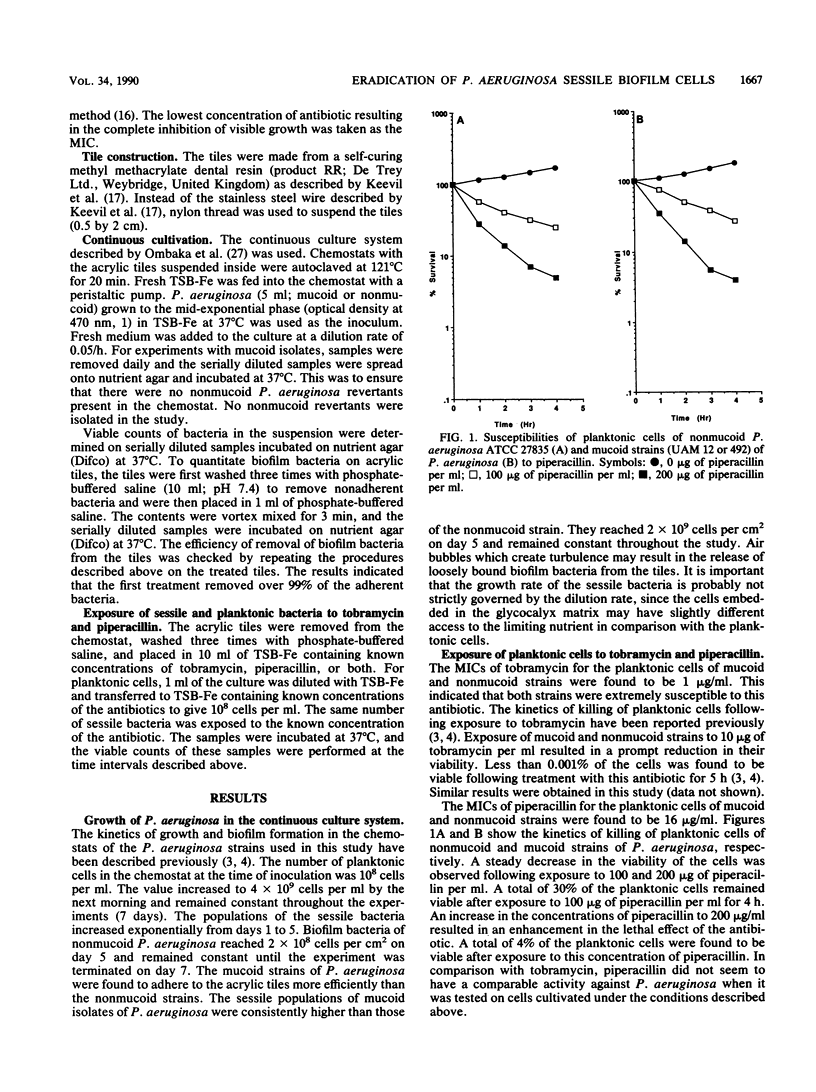
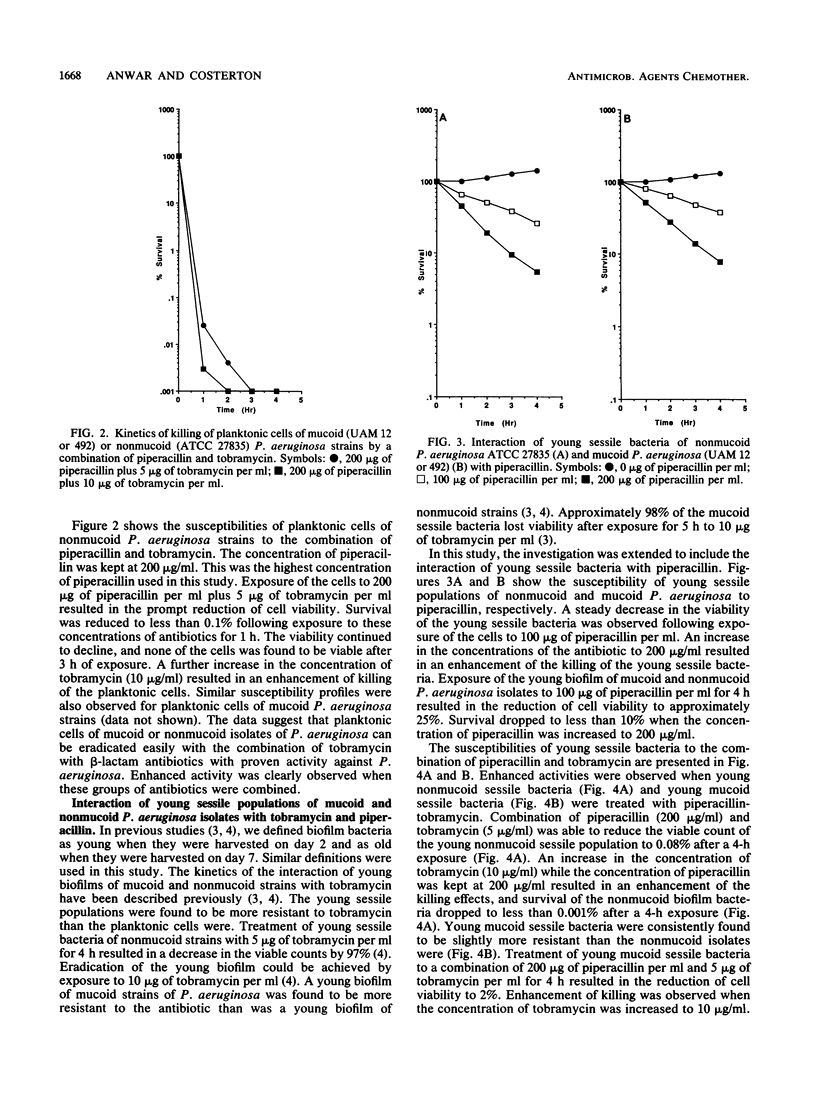
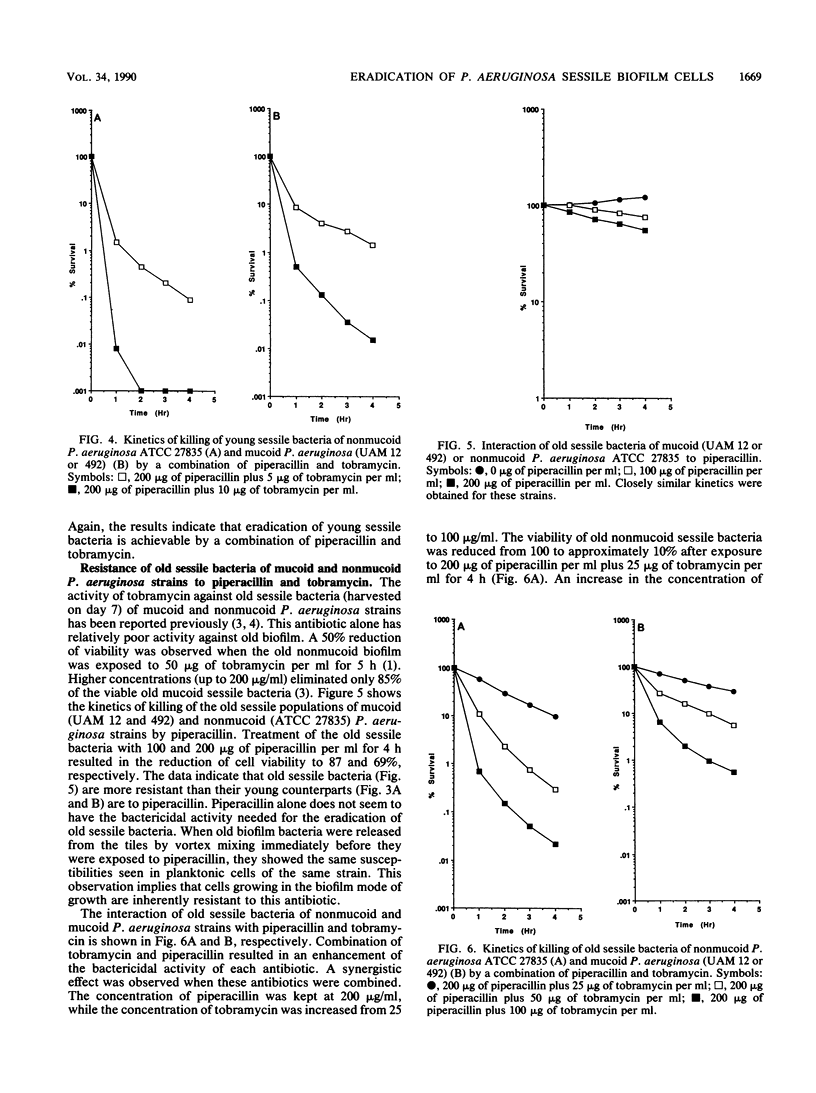
An in vitro chemostat system in which Pseudomonas aeruginosa can be cultivated at a slow growth rate and under iron limitation conditions was used to study the susceptibilities of sessile bacteria of mucoid and nonmucoid P. aeruginosa strains to tobramycin and piperacillin. Planktonic cells of both mucoid and nonmucoid P. aeruginosa strains were susceptible to tobramycin and piperacillin. None of the cells was found to be viable after 2 h of exposure to 200 micrograms of piperacillin plus 10 micrograms of tobramycin per ml. Young sessile bacteria were slightly more resistant to piperacillin or tobramycin than the planktonic cells were. However, eradication of young sessile bacteria could be achieved with a combination of piperacillin and tobramycin. None of these young biofilm bacteria were found to be viable after a 2-h exposure to 200 micrograms of piperacillin plus 10 micrograms of tobramycin per ml. Old sessile bacteria were very resistant to these antibiotics. Eradication of old sessile bacteria could not be achieved with either tobramycin (200 micrograms/ml) or piperacillin (200 micrograms/ml) alone. Combination of higher concentrations of tobramycin with piperacillin resulted in an enhancement of killing of the old sessile bacteria. Exposure of old sessile bacteria to 200 micrograms of piperacillin plus 100 micrograms of tobramycin per ml resulted in the reduction of the viable count to approximately 0.02%. The data suggest that the eradication of biofilm-associated infections is best carried out as early as possible. Enhanced activities against the sessile bacteria were achieved when higher concentrations of aminoglycosides were combined with beta-lactam antibiotics.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar H., Brown M. R., Lambert P. A. Effect of nutrient depletion on sensitivity of Pseudomonas cepacia to phagocytosis and serum bactericidal activity at different temperatures. J Gen Microbiol. 1983 Jul;129(7):2021–2027. doi: 10.1099/00221287-129-7-2021. [DOI] [PubMed] [Google Scholar]
- Anwar H., Dasgupta M., Lam K., Costerton J. W. Tobramycin resistance of mucoid Pseudomonas aeruginosa biofilm grown under iron limitation. J Antimicrob Chemother. 1989 Nov;24(5):647–655. doi: 10.1093/jac/24.5.647. [DOI] [PubMed] [Google Scholar]
- Anwar H., van Biesen T., Dasgupta M., Lam K., Costerton J. W. Interaction of biofilm bacteria with antibiotics in a novel in vitro chemostat system. Antimicrob Agents Chemother. 1989 Oct;33(10):1824–1826. doi: 10.1128/aac.33.10.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Williams P. The influence of environment on envelope properties affecting survival of bacteria in infections. Annu Rev Microbiol. 1985;39:527–556. doi: 10.1146/annurev.mi.39.100185.002523. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Lam J., Lam K., Chan R. The role of the microcolony mode of growth in the pathogenesis of Pseudomonas aeruginosa infections. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S867–S873. doi: 10.1093/clinids/5.supplement_5.s867. [DOI] [PubMed] [Google Scholar]
- Costerton J. W. Structure and plasticity at various organization levels in the bacterial cell. Can J Microbiol. 1988 Apr;34(4):513–521. doi: 10.1139/m88-088. [DOI] [PubMed] [Google Scholar]
- Kadurugamuwa J. L., Anwar H., Brown M. R., Shand G. H., Ward K. H. Media for study of growth kinetics and envelope properties of iron-deprived bacteria. J Clin Microbiol. 1987 May;25(5):849–855. doi: 10.1128/jcm.25.5.849-855.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keevil C. W., Bradshaw D. J., Dowsett A. B., Feary T. W. Microbial film formation: dental plaque deposition on acrylic tiles using continuous culture techniques. J Appl Bacteriol. 1987 Feb;62(2):129–138. doi: 10.1111/j.1365-2672.1987.tb02390.x. [DOI] [PubMed] [Google Scholar]
- Klastersky J., Cappel R., Daneau D. Clinical significance of in vitro synergism between antibiotics in gram-negative infections. Antimicrob Agents Chemother. 1972 Dec;2(6):470–475. doi: 10.1128/aac.2.6.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunin C. M., Steele C. Culture of the surfaces of urinary catheters to sample urethral flora and study the effect of antimicrobial therapy. J Clin Microbiol. 1985 Jun;21(6):902–908. doi: 10.1128/jcm.21.6.902-908.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols W. W., Evans M. J., Slack M. P., Walmsley H. L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989 May;135(5):1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- Nickel J. C., Gristina A. G., Costerton J. W. Electron microscopic study of an infected Foley catheter. Can J Surg. 1985 Jan;28(1):50-1, 54. [PubMed] [Google Scholar]
- Nickel J. C., Heaton J., Morales A., Costerton J. W. Bacterial biofilm in persistent penile prosthesis-associated infection. J Urol. 1986 Mar;135(3):586–588. doi: 10.1016/s0022-5347(17)45747-8. [DOI] [PubMed] [Google Scholar]
- Nickel J. C., Ruseska I., Wright J. B., Costerton J. W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985 Apr;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombaka E. A., Cozens R. M., Brown M. R. Influence of nutrient limitation of growth on stability and production of virulence factors of mucoid and nonmucoid strains of Pseudomonas aeruginosa. Rev Infect Dis. 1983 Nov-Dec;5 (Suppl 5):S880–S888. doi: 10.1093/clinids/5.supplement_5.s880. [DOI] [PubMed] [Google Scholar]
- Prosser B. L., Taylor D., Dix B. A., Cleeland R. Method of evaluating effects of antibiotics on bacterial biofilm. Antimicrob Agents Chemother. 1987 Oct;31(10):1502–1506. doi: 10.1128/aac.31.10.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand G. H., Anwar H., Brown M. R. Outer membrane proteins of polymyxin resistant Pseudomonas aeruginosa: effect of magnesium depletion. J Antimicrob Chemother. 1988 Dec;22(6):811–821. doi: 10.1093/jac/22.6.811. [DOI] [PubMed] [Google Scholar]
- Shand G. H., Anwar H., Kadurugamuwa J., Brown M. R., Silverman S. H., Melling J. In vivo evidence that bacteria in urinary tract infection grow under iron-restricted conditions. Infect Immun. 1985 Apr;48(1):35–39. doi: 10.1128/iai.48.1.35-39.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slack M. P., Nichols W. W. Antibiotic penetration through bacterial capsules and exopolysaccharides. J Antimicrob Chemother. 1982 Nov;10(5):368–372. doi: 10.1093/jac/10.5.368. [DOI] [PubMed] [Google Scholar]
- Slack M. P., Nichols W. W. The penetration of antibiotics through sodium alginate and through the exopolysaccharide of a mucoid strain of Pseudomonas aeruginosa. Lancet. 1981 Sep 5;2(8245):502–503. doi: 10.1016/s0140-6736(81)90885-0. [DOI] [PubMed] [Google Scholar]
- Ward K. H., Anwar H., Brown R. W., Wale J., Gowar J. Antibody response to outer-membrane antigens of Pseudomonas aeruginosa in human burn wound infection. J Med Microbiol. 1988 Nov;27(3):179–190. doi: 10.1099/00222615-27-3-179. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Muncie H. L., Jr, Bergquist E. J., Hoopes J. M. Sequelae and management of urinary infection in the patient requiring chronic catheterization. J Urol. 1981 Jan;125(1):1–8. doi: 10.1016/s0022-5347(17)54874-0. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and infection. Microbiol Rev. 1978 Mar;42(1):45–66. doi: 10.1128/mr.42.1.45-66.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. D. Iron withholding: a defense against infection and neoplasia. Physiol Rev. 1984 Jan;64(1):65–102. doi: 10.1152/physrev.1984.64.1.65. [DOI] [PubMed] [Google Scholar]


