Abstract
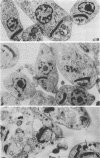
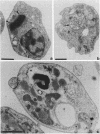
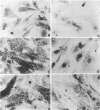
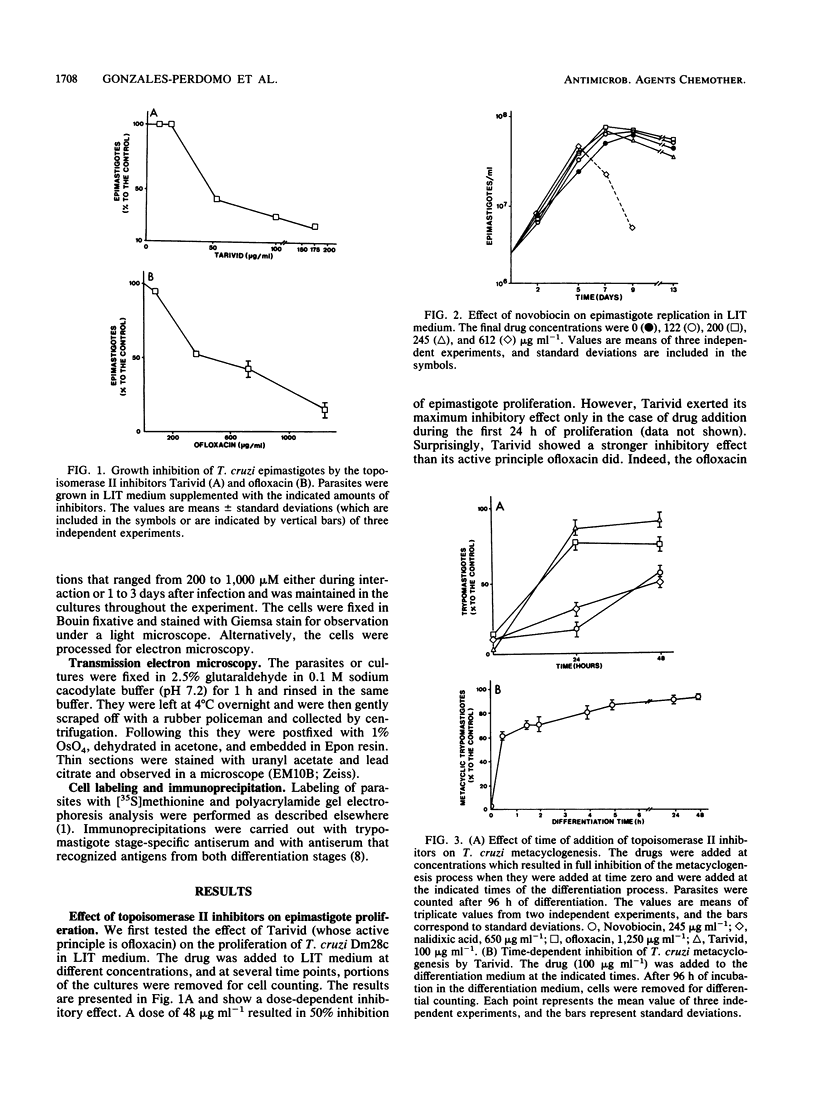
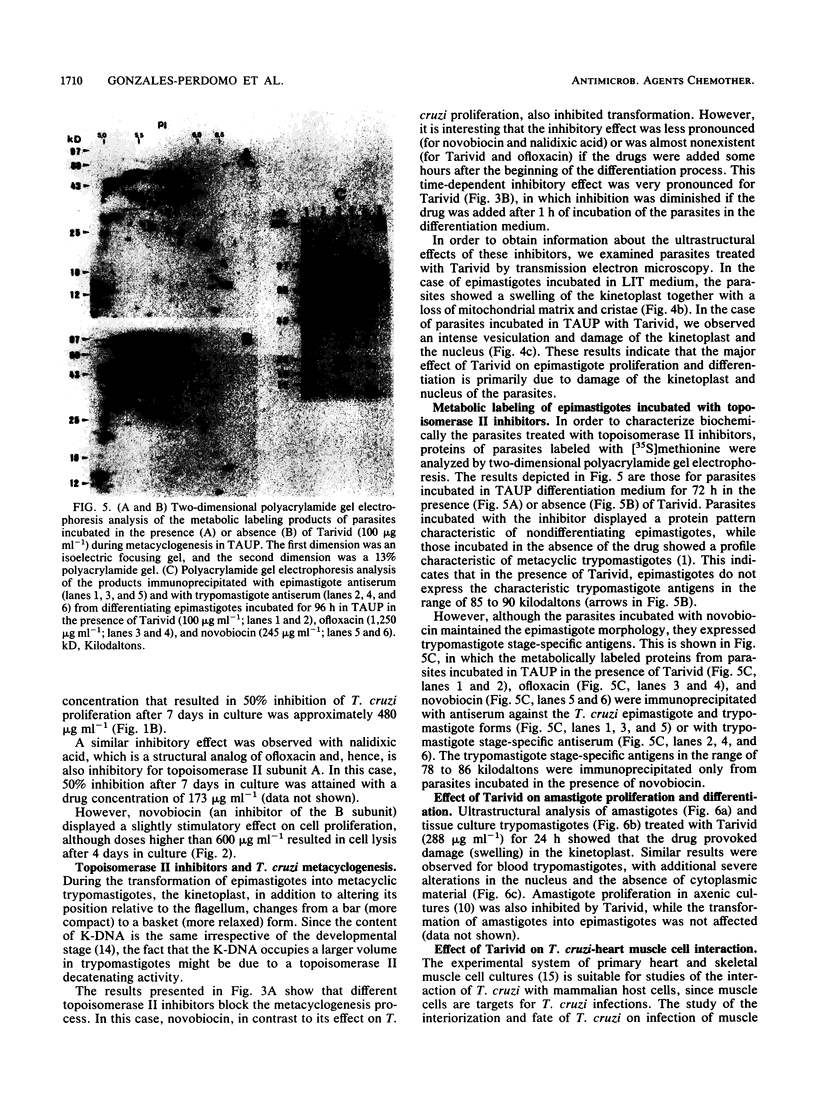
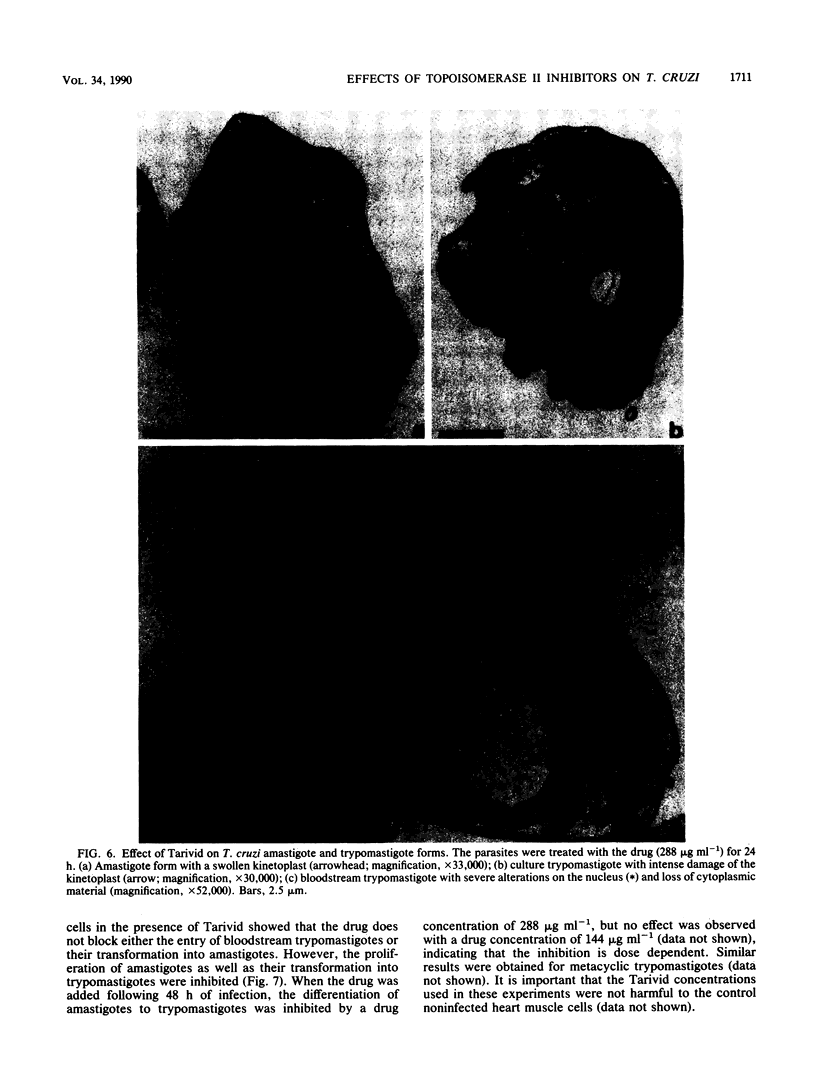
Bacterial topoisomerase II inhibitors (ofloxacin and its commercial derivative Tarivid, nalidixic acid, and novobiocin) were tested as blockers of Trypanosoma cruzi differentiation and proliferation. The transformation of either epimastigotes into metacyclic trypomastigotes or amastigotes into trypomastigotes was inhibited by the drugs in a dose-dependent manner. The inhibition of epimastigote differentiation was also dependent on the time of drug addition to the medium. Proliferation of T. cruzi was also blocked in a dose-dependent manner by the drugs, with the exception of novobiocin, which did not inhibit epimastigote replication and resulted in cell lysis when it was used at high concentrations. On the other hand, the transformation of amastigotes into epimastigotes in axenic culture was not inhibited; this process did not require either kinetoplast (mitochondrial) DNA replication or changes in the DNA network organization. Electron microscopy of cells treated with Tarivid (ofloxacin) showed damage to the kinetoplast, suggesting that this organelle might be the target of the drug. These results indicate that a bacterial-like topoisomerase II plays an important role in T. cruzi proliferation and differentiation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonaldo M. C., Souto-Padron T., de Souza W., Goldenberg S. Cell-substrate adhesion during Trypanosoma cruzi differentiation. J Cell Biol. 1988 Apr;106(4):1349–1358. doi: 10.1083/jcb.106.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMARGO E. P. GROWTH AND DIFFERENTIATION IN TRYPANOSOMA CRUZI. I. ORIGIN OF METACYCLIC TRYPANOSOMES IN LIQUID MEDIA. Rev Inst Med Trop Sao Paulo. 1964 May-Jun;6:93–100. [PubMed] [Google Scholar]
- Chakraborty A. K., Majumder H. K. Decatenation of kinetoplast DNA by an ATP-dependent DNA topoisomerase from the kinetoplast hemoflagellate Leishmania donovani. Mol Biochem Parasitol. 1987 Dec;26(3):215–224. doi: 10.1016/0166-6851(87)90074-0. [DOI] [PubMed] [Google Scholar]
- Contreras V. T., Araujo-Jorge T. C., Bonaldo M. C., Thomaz N., Barbosa H. S., Meirelles M. de N., Goldenberg S. Biological aspects of the Dm 28c clone of Trypanosoma cruzi after metacyclogenesis in chemically defined media. Mem Inst Oswaldo Cruz. 1988 Jan-Mar;83(1):123–133. doi: 10.1590/s0074-02761988000100016. [DOI] [PubMed] [Google Scholar]
- Contreras V. T., Morel C. M., Goldenberg S. Stage specific gene expression precedes morphological changes during Trypanosoma cruzi metacyclogenesis. Mol Biochem Parasitol. 1985 Jan;14(1):83–96. doi: 10.1016/0166-6851(85)90108-2. [DOI] [PubMed] [Google Scholar]
- Contreras V. T., Salles J. M., Thomas N., Morel C. M., Goldenberg S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol Biochem Parasitol. 1985 Sep;16(3):315–327. doi: 10.1016/0166-6851(85)90073-8. [DOI] [PubMed] [Google Scholar]
- Croft S. L., Hogg J. Limited activity of bacterial DNA topoisomerase II inhibitors against Leishmania donovani and Trypanosoma cruzi amastigotes in vitro. Trans R Soc Trop Med Hyg. 1988;82(6):856–856. doi: 10.1016/0035-9203(88)90017-x. [DOI] [PubMed] [Google Scholar]
- Dias J. C. Control of Chagas disease in Brazil. Parasitol Today. 1987 Nov;3(11):336–341. doi: 10.1016/0169-4758(87)90117-7. [DOI] [PubMed] [Google Scholar]
- Douc-Rasy S., Kayser A., Riou J. F., Riou G. ATP-independent type II topoisomerase from trypanosomes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7152–7156. doi: 10.1073/pnas.83.19.7152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvorak J. A., Hall T. E., Crane M. S., Engel J. C., McDaniel J. P., Uriegas R. Trypanosoma cruzi: flow cytometric analysis. I. Analysis of total DNA/organism by means of mithramycin-induced fluorescence. J Protozool. 1982 Aug;29(3):430–437. doi: 10.1111/j.1550-7408.1982.tb05427.x. [DOI] [PubMed] [Google Scholar]
- Mackenstedt U., Brockelman C. R., Mehlhorn H., Raether W. Comparative morphology of human and animal malaria parasites. I. Host-parasite interface. Parasitol Res. 1989;75(7):528–535. doi: 10.1007/BF00931161. [DOI] [PubMed] [Google Scholar]
- Meirelles M. N., de Araujo-Jorge T. C., Miranda C. F., de Souza W., Barbosa H. S. Interaction of Trypanosoma cruzi with heart muscle cells: ultrastructural and cytochemical analysis of endocytic vacuole formation and effect upon myogenesis in vitro. Eur J Cell Biol. 1986 Aug;41(2):198–206. [PubMed] [Google Scholar]
- Melendy T., Sheline C., Ray D. S. Localization of a type II DNA topoisomerase to two sites at the periphery of the kinetoplast DNA of Crithidia fasciculata. Cell. 1988 Dec 23;55(6):1083–1088. doi: 10.1016/0092-8674(88)90252-8. [DOI] [PubMed] [Google Scholar]
- Shapiro T. A., Klein V. A., Englund P. T. Drug-promoted cleavage of kinetoplast DNA minicircles. Evidence for type II topoisomerase activity in trypanosome mitochondria. J Biol Chem. 1989 Mar 5;264(7):4173–4178. [PubMed] [Google Scholar]
- Shlomai J., Zadok A., Frank D. A unique ATP-dependent DNA topoisomerase from trypanosomatids. Adv Exp Med Biol. 1984;179:409–422. doi: 10.1007/978-1-4684-8730-5_42. [DOI] [PubMed] [Google Scholar]
- de Carvalho T. U., de Souza W. Separation of amastigotes and trypomastigotes of Trypanosoma cruzi from cultured cells. Z Parasitenkd. 1983;69(5):571–575. doi: 10.1007/BF00926668. [DOI] [PubMed] [Google Scholar]
- de Castro S. L., Meirelles M. de N., Oliveira M. M. Trypanosoma cruzi: adrenergic modulation of cyclic AMP role in proliferation and differentiation of amastigotes in vitro. Exp Parasitol. 1987 Dec;64(3):368–375. doi: 10.1016/0014-4894(87)90049-x. [DOI] [PubMed] [Google Scholar]
- de Souza W. Cell biology of Trypanosoma cruzi. Int Rev Cytol. 1984;86:197–283. doi: 10.1016/s0074-7696(08)60180-1. [DOI] [PubMed] [Google Scholar]