Abstract
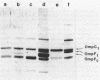
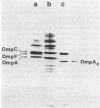
We studied a clinical isolate of Salmonella typhi (strain 1895) characterized by resistance to 200 micrograms of chloramphenicol per ml despite the absence of chloramphenicol-inactivating activity. The outer membrane protein profile analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis indicated a deficiency of one of the major protein species which may serve as a porin for entry of chloramphenicol. When the strain was transformed with a plasmid encoding chloramphenicol acetyltransferase, chloramphenicol added to the culture was not inactivated, suggesting a drastic reduction of permeability towards the drug. Moreover, transformants bearing a plasmid coding for the Escherichia coli OmpF porin became considerably more susceptible to chloramphenicol (40 micrograms/ml). On the other hand, transformants carrying a plasmid encoding the Salmonella typhi ompC gene remained as resistant to the drug as the parental strain, even though they overexpressed OmpC. These findings indicate that the lack of OmpF plays a major role in the resistance to chloramphenicol in strain 1895.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agüero J., Mora G., Mroczenski-Wildey M. J., Fernandez-Beros M. E., Aron L., Cabello F. C. Cloning, expression and characterization of the 36 KDal Salmonella typhi porin gene in Escherichia coli. Microb Pathog. 1987 Dec;3(6):399–407. doi: 10.1016/0882-4010(87)90010-6. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns J. L., Mendelman P. M., Levy J., Stull T. L., Smith A. L. A permeability barrier as a mechanism of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1985 Jan;27(1):46–54. doi: 10.1128/aac.27.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon I., Lobos S. R., Mora G. C. The hemolytic effect of Salmonella typhi Ty 2 porins. Eur J Biochem. 1984 Jun 15;141(3):579–583. doi: 10.1111/j.1432-1033.1984.tb08232.x. [DOI] [PubMed] [Google Scholar]
- Chopra I., Eccles S. J. Diffusion of tetracycline across the outer membrane of Escherichia coli K-12: involvement of protein Ia. Biochem Biophys Res Commun. 1978 Jul 28;83(2):550–557. doi: 10.1016/0006-291x(78)91025-2. [DOI] [PubMed] [Google Scholar]
- Gaffney D. F., Cundliffe E., Foster T. J. Chloramphenicol resistance that does not involve chloramphenicol acetyltransferase encoded by plasmids from gram-negative bacteria. J Gen Microbiol. 1981 Jul;125(1):113–121. doi: 10.1099/00221287-125-1-113. [DOI] [PubMed] [Google Scholar]
- Goldstein F. W., Gutmann L., Williamson R., Collatz E., Acar J. F. In vivo and in vitro emergence of simultaneous resistance to both beta-lactam and aminoglycoside antibiotics in a strain of Serratia marcescens. Ann Microbiol (Paris) 1983 May-Jun;134A(3):329–337. [PubMed] [Google Scholar]
- Gutmann L., Williamson R., Moreau N., Kitzis M. D., Collatz E., Acar J. F., Goldstein F. W. Cross-resistance to nalidixic acid, trimethoprim, and chloramphenicol associated with alterations in outer membrane proteins of Klebsiella, Enterobacter, and Serratia. J Infect Dis. 1985 Mar;151(3):501–507. doi: 10.1093/infdis/151.3.501. [DOI] [PubMed] [Google Scholar]
- Harder K. J., Nikaido H., Matsuhashi M. Mutants of Escherichia coli that are resistant to certain beta-lactam compounds lack the ompF porin. Antimicrob Agents Chemother. 1981 Oct;20(4):549–552. doi: 10.1128/aac.20.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin R. T., MacAlister T. J., Chan R., Costerton J. W. Citrate-tris(hydroxymethyl)aminomethane-mediated release of outer membrane sections from the cell envelope of a deep-rough (heptose-deficient lipopolysaccharide) strain of Escherichia coli O8. J Bacteriol. 1981 Mar;145(3):1386–1396. doi: 10.1128/jb.145.3.1386-1396.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe A., Chabbert Y. A., Semonin O. Role of porin proteins OmpF and OmpC in the permeation of beta-lactams. Antimicrob Agents Chemother. 1982 Dec;22(6):942–948. doi: 10.1128/aac.22.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kado C. I., Liu S. T. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981 Mar;145(3):1365–1373. doi: 10.1128/jb.145.3.1365-1373.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labarca P., Lobos S., Calderón I., Mora G. Native and chemically modified porin channels from Salmonella typhi Ty2 in planar lipid bilayers. FEBS Lett. 1986 Mar 3;197(1-2):211–216. doi: 10.1016/0014-5793(86)80328-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Medeiros A. A., O'Brien T. F., Rosenberg E. Y., Nikaido H. Loss of OmpC porin in a strain of Salmonella typhimurium causes increased resistance to cephalosporins during therapy. J Infect Dis. 1987 Nov;156(5):751–757. doi: 10.1093/infdis/156.5.751. [DOI] [PubMed] [Google Scholar]
- Mizuno T., Kasai H., Mizushima S. Construction of a series of ompC-ompF chimeric genes by in vivo homologous recombination in Escherichia coli and characterization of their translational products. Mol Gen Genet. 1987 May;207(2-3):217–223. doi: 10.1007/BF00331581. [DOI] [PubMed] [Google Scholar]
- Nakae T. Identification of the outer membrane protein of E. coli that produces transmembrane channels in reconstituted vesicle membranes. Biochem Biophys Res Commun. 1976 Aug 9;71(3):877–884. doi: 10.1016/0006-291x(76)90913-x. [DOI] [PubMed] [Google Scholar]
- Nakae T. Outer-membrane permeability of bacteria. Crit Rev Microbiol. 1986;13(1):1–62. doi: 10.3109/10408418609108734. [DOI] [PubMed] [Google Scholar]
- Nierhaus K. H., Dohme F. Total reconstitution of 50 S subunits from Escherichia coli ribosomes. Methods Enzymol. 1979;59:443–449. doi: 10.1016/0076-6879(79)59106-x. [DOI] [PubMed] [Google Scholar]
- Nikaido H. Outer membrane of Salmonella typhimurium. Transmembrane diffusion of some hydrophobic substances. Biochim Biophys Acta. 1976 Apr 16;433(1):118–132. doi: 10.1016/0005-2736(76)90182-6. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Rosenberg E. Y., Foulds J. Porin channels in Escherichia coli: studies with beta-lactams in intact cells. J Bacteriol. 1983 Jan;153(1):232–240. doi: 10.1128/jb.153.1.232-240.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto S., Suzuki Y. Chloramphenicol-, dihydrostreptomycin-, and kanamycin-inactivating enzymes from multiple drug-resistant Escherichia coli carrying episome 'R'. Nature. 1965 Dec 25;208(5017):1301–1303. doi: 10.1038/2081301a0. [DOI] [PubMed] [Google Scholar]
- Preheim L. C., Penn R. G., Sanders C. C., Goering R. V., Giger D. K. Emergence of resistance to beta-lactam and aminoglycoside antibiotics during moxalactam therapy of Pseudomonas aeruginosa infections. Antimicrob Agents Chemother. 1982 Dec;22(6):1037–1041. doi: 10.1128/aac.22.6.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr Microbial resistance to newer generation beta-lactam antibiotics: clinical and laboratory implications. J Infect Dis. 1985 Mar;151(3):399–406. doi: 10.1093/infdis/151.3.399. [DOI] [PubMed] [Google Scholar]
- Sawai T., Hiruma R., Kawana N., Kaneko M., Taniyasu F., Inami A. Outer membrane permeation of beta-lactam antibiotics in Escherichia coli, Proteus mirabilis, and Enterobacter cloacae. Antimicrob Agents Chemother. 1982 Oct;22(4):585–592. doi: 10.1128/aac.22.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Solubilization of the cytoplasmic membrane of Escherichia coli by Triton X-100. J Bacteriol. 1971 Oct;108(1):545–552. doi: 10.1128/jb.108.1.545-552.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venegas A., Gómez I., Zaror I., Yudelevich A. The nucleotide sequence of the Salmonella typhi ompC porin gene. Nucleic Acids Res. 1988 Aug 11;16(15):7721–7721. doi: 10.1093/nar/16.15.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaror I., Gómez I., Castillo G., Yudelevich A., Venegas A. Molecular cloning and expression in E. coli of a Salmonella typhi porin gene. FEBS Lett. 1988 Feb 29;229(1):77–81. doi: 10.1016/0014-5793(88)80801-9. [DOI] [PubMed] [Google Scholar]