Abstract
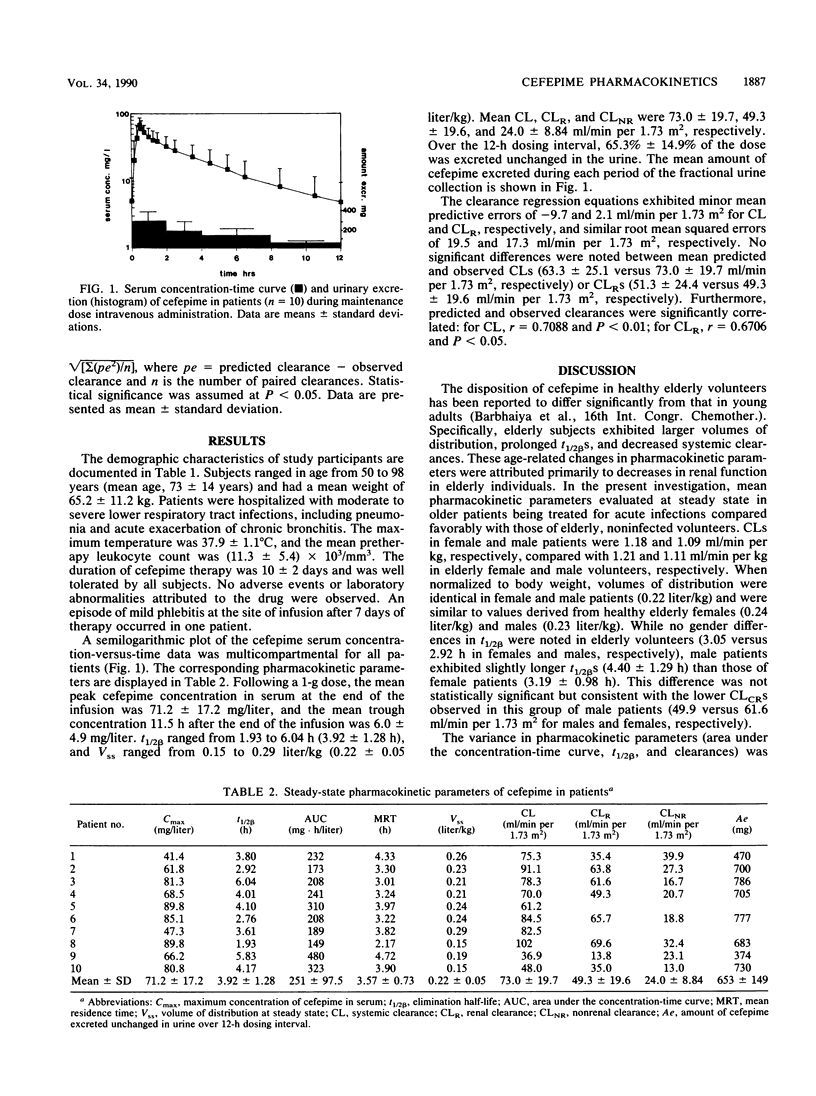
The steady-state pharmacokinetics of cefepime were evaluated in 10 middle-aged and elderly patients with acute lower respiratory tract infections who were receiving 1 g intravenously every 12 h. One preinfusion and 15 postinfusion serum samples and total urine output were collected over one dosing interval between days 3 and 8 of therapy. Cefepime concentrations in serum over time exhibited a multicompartmental profile. Peak and trough concentrations in serum determined by a validated high-performance liquid chromatography method were 71.2 +/- 17.2 (mean +/- standard deviation) and 6.0 +/- 4.9 mg/liter, respectively. The steady-state volume of distribution was 0.22 +/- 0.05 liter/kg. Elimination half-lives ranged from 1.93 to 6.04 h (3.92 +/- 1.28 h), and total body clearances ranged from 36.9 to 102 ml/min per 1.73 m2 (73.0 +/- 19.7 ml/min per 1.73 m2). The disposition of cefepime at steady state in patients was comparable to previous observations in healthy elderly volunteers. The predictive performance of regression equations derived from single-dose studies in volunteers relating creatinine clearance with total body and renal clearances of cefepime exhibited slight biases (mean predictive errors, -9.7 and 2.1 ml/min per 1.73 m2, respectively) and similar precisions. Predicted and observed total body clearances (63.3 +/- 25.1 versus 73.0 +/- 19.7 ml/min per 1.73 m2, respectively) and renal clearances (51.3 +/- 24.4 versus 49.3 +/- 19.6 ml/min per 1.73 m2, respectively) were not significantly different. The pharmacokinetics of cefepime in infected patients appeared to be unaltered by illness, and the steady-state disposition of cefepime was predictable from data derived from single-dose studies in volunteers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbhaiya R. H., Forgue S. T., Shyu W. C., Papp E. A., Pittman K. A. High-pressure liquid chromatographic analysis of BMY-28142 in plasma and urine. Antimicrob Agents Chemother. 1987 Jan;31(1):55–59. doi: 10.1128/aac.31.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer L. A., Gibaldi M. Computation of model-independent pharmacokinetic parameters during multiple dosing. J Pharm Sci. 1983 Aug;72(8):978–979. doi: 10.1002/jps.2600720843. [DOI] [PubMed] [Google Scholar]
- Bianco T. M., Dwyer P. N., Bertino J. S., Jr Gentamicin pharmacokinetics, nephrotoxicity, and prediction of mortality in febrile neutropenic patients. Antimicrob Agents Chemother. 1989 Nov;33(11):1890–1895. doi: 10.1128/aac.33.11.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockcroft D. W., Gault M. H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Drusano G. L., Plaisance K. I., Forrest A., Bustamante C., Devlin A., Standiford H. C., Wade J. C. Steady-state pharmacokinetics of imipenem in febrile neutropenic cancer patients. Antimicrob Agents Chemother. 1987 Sep;31(9):1420–1422. doi: 10.1128/aac.31.9.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C., Jones R. N., Barry A. L., Thornsberry C. Evaluation of the in vitro activity of BMY-28142, a new broad-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 May;27(5):679–682. doi: 10.1128/aac.27.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganzinger U., Kleinberger G., Lenz K., Schatz F., Laggner A., Grimm G. Pharmacokinetics of ceftazidime and netilmicin in patients with sepsis. Int J Clin Pharmacol Ther Toxicol. 1987 Jul;25(7):354–362. [PubMed] [Google Scholar]
- Graves D. A. Failure of single-dose kinetics to predict steady state. Drug Intell Clin Pharm. 1988 Nov;22(11):917–918. doi: 10.1177/106002808802201128. [DOI] [PubMed] [Google Scholar]
- Heim-Duthoy K. L., Bubrick M. P., Cocchetto D. M., Matzke G. R. Disposition of ceftazidime in surgical patients with intra-abdominal infection. Antimicrob Agents Chemother. 1988 Dec;32(12):1845–1847. doi: 10.1128/aac.32.12.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim-Duthoy K. L., Peltier G. L., Guay D. R., Matzke G. R. Pharmacokinetics of cefonicid in patients with skin and skin structure infections. Antimicrob Agents Chemother. 1988 Apr;32(4):485–487. doi: 10.1128/aac.32.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata C. A., Guay D. R., Awni W. M., Stein D. J., Peterson P. K. Steady-state pharmacokinetics of intravenous and oral ciprofloxacin in elderly patients. Antimicrob Agents Chemother. 1989 Nov;33(11):1927–1931. doi: 10.1128/aac.33.11.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke D. M., Cafarell R. F., Parker S. W., Apicella M. A., Jusko W. J. Pharmacokinetics of aztreonam in patients with gram-negative infections. Antimicrob Agents Chemother. 1985 Jan;27(1):16–20. doi: 10.1128/aac.27.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nye K. J., Shi Y. G., Andrews J. M., Wise R. Pharmacokinetics and tissue penetration of cefepime. J Antimicrob Chemother. 1989 Jul;24(1):23–28. doi: 10.1093/jac/24.1.23. [DOI] [PubMed] [Google Scholar]
- Visser M. R., Hoepelman I. M., Beumer H., Rozenberg-Arska M., Verhoef J. Comparative in vitro antibacterial activity of the new carbapenem meropenem (SM-7338). Eur J Clin Microbiol Infect Dis. 1989 Dec;8(12):1061–1064. doi: 10.1007/BF01975170. [DOI] [PubMed] [Google Scholar]
- Vuye A., Pijck J. In vitro antibacterial activity of BMY-28142, a new extended-spectrum cephalosporin. Antimicrob Agents Chemother. 1985 Apr;27(4):574–577. doi: 10.1128/aac.27.4.574. [DOI] [PMC free article] [PubMed] [Google Scholar]



