Abstract
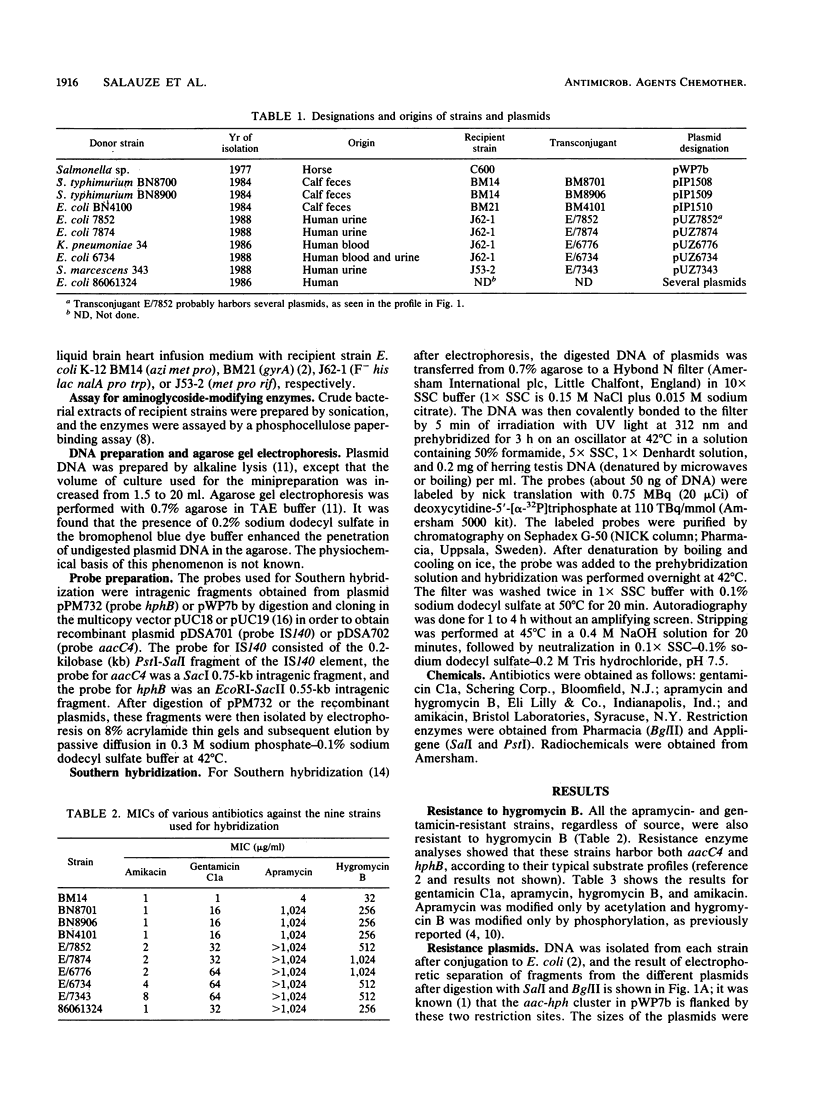
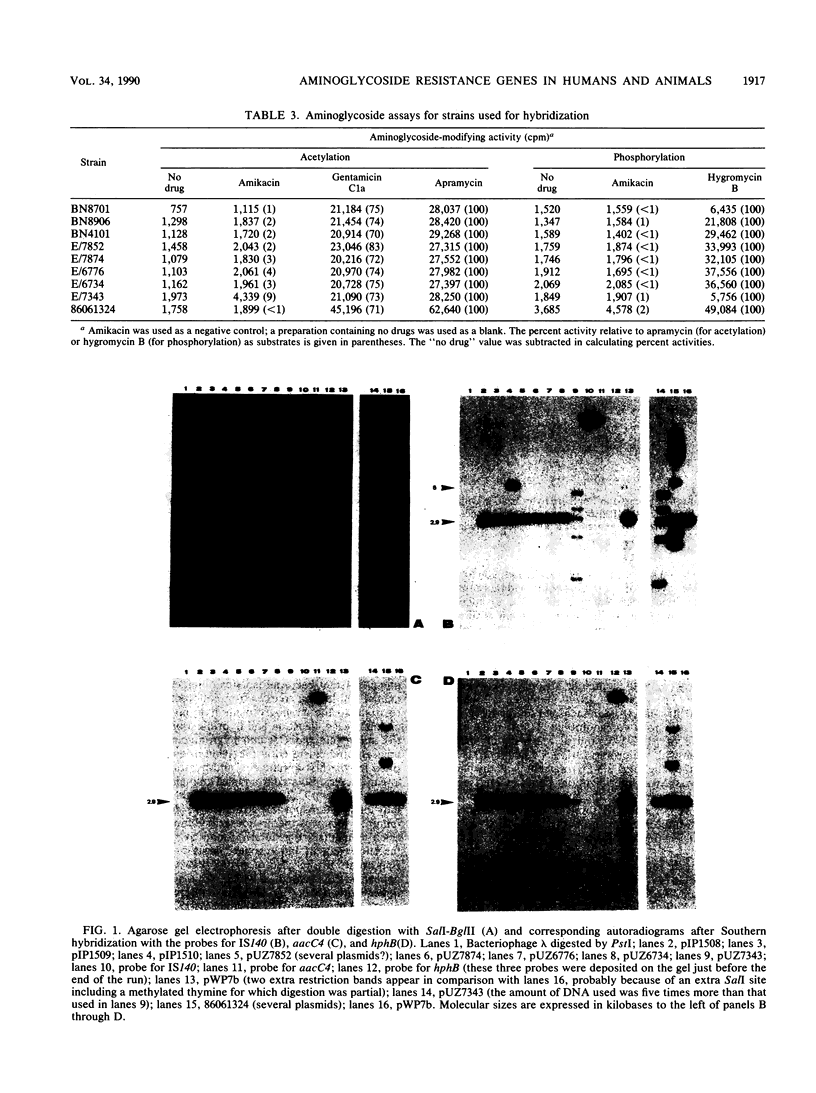
Members of the family Enterobacteriaceae harboring an enzyme of the aminoglycoside acetyltransferase 3 class (AAC-3-IV) (apramycin and gentamicin resistance) and hygromycin B phosphotransferase 4 (HPH-4-I) (hygromycin B resistance) have been isolated from human clinical sources in Europe. A cluster of genes containing IS140, aacC4, and hphB was found in these strains. We demonstrate by Southern hybridization that this cluster is identical to the operon found in animals that also contains insertion sequences belonging to the ISO family. This provides another example of presumptive transfer of antibiotic resistance genes between bacteria of animal and human origin.
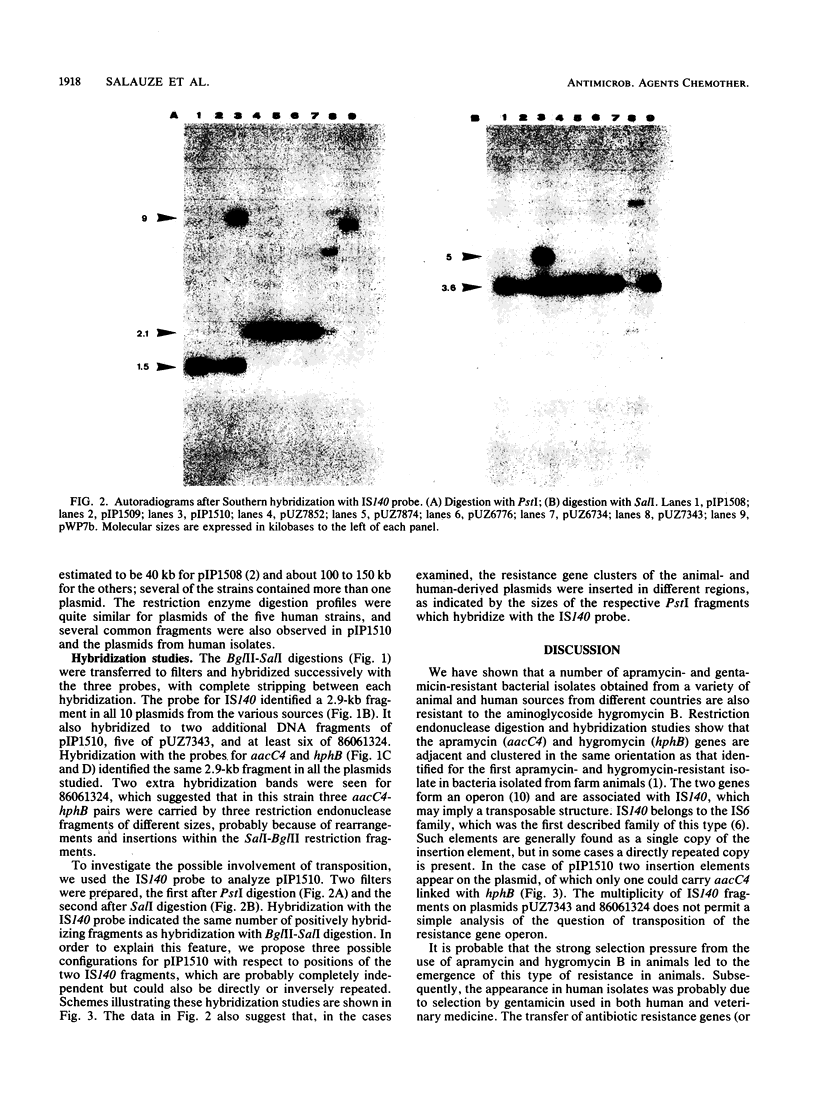
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bräu B., Pilz U., Piepersberg W. Genes for gentamicin-(3)-N-acetyltransferases III and IV: I. Nucleotide sequence of the AAC(3)-IV gene and possible involvement of an IS140 element in its expression. Mol Gen Genet. 1984;193(1):179–187. doi: 10.1007/BF00327434. [DOI] [PubMed] [Google Scholar]
- Chaslus-Dancla E., Martel J. L., Carlier C., Lafont J. P., Courvalin P. Emergence of aminoglycoside 3-N-acetyltransferase IV in Escherichia coli and Salmonella typhimurium isolated from animals in France. Antimicrob Agents Chemother. 1986 Feb;29(2):239–243. doi: 10.1128/aac.29.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., O'Connor S. Enzymatic modification of aminoglycoside antibiotics: 3-N-acetyltransferase with broad specificity that determines resistance to the novel aminoglycoside apramycin. Antimicrob Agents Chemother. 1978 Jul;14(1):69–72. doi: 10.1128/aac.14.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Haas M. J., Dowding J. E. Aminoglycoside-modifying enzymes. Methods Enzymol. 1975;43:611–628. doi: 10.1016/0076-6879(75)43124-x. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Shannon K. P. Resistance to apramycin in Escherichia coli isolated from animals: detection of a novel aminoglycoside-modifying enzyme. J Gen Microbiol. 1984 Mar;130(3):473–482. doi: 10.1099/00221287-130-3-473. [DOI] [PubMed] [Google Scholar]
- Kaster K. R., Burgett S. G., Rao R. N., Ingolia T. D. Analysis of a bacterial hygromycin B resistance gene by transcriptional and translational fusions and by DNA sequencing. Nucleic Acids Res. 1983 Oct 11;11(19):6895–6911. doi: 10.1093/nar/11.19.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien T. F., Hopkins J. D., Gilleece E. S., Medeiros A. A., Kent R. L., Blackburn B. O., Holmes M. B., Reardon J. P., Vergeront J. M., Schell W. L. Molecular epidemiology of antibiotic resistance in salmonella from animals and human beings in the United States. N Engl J Med. 1982 Jul 1;307(1):1–6. doi: 10.1056/NEJM198207013070101. [DOI] [PubMed] [Google Scholar]
- Piepersberg W., Bräu B., Davies J. High frequency transduction of R-factor encoded gentamicin resistance by bacteriophage P1Cm. J Antibiot (Tokyo) 1983 May;36(5):588–594. doi: 10.7164/antibiotics.36.588. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961 May;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]