Abstract
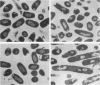
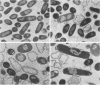
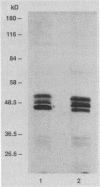
Trospectomycin sulfate, a chemically synthesized analog of spectinomycin, exhibits a broad range of activity against both aerobes and anaerobes, including the etiological agents of sexually transmitted diseases. Its activity in vitro against Escherichia coli is considered only moderate. At subinhibitory levels, however, trospectomycin induced changes in a pathogenic strain of E. coli, UC 9451, which significantly increased its sensitivity to serum lysis. This strain of E. coli shows high-level resistance to serum in vitro, typically growing twofold within a 45-min incubation period. Following exposure to one-fifth the MIC of trospectomycin, greater than 99% of the bacteria were killed in 25% serum within 15 min. Surviving bacteria were static in this level of serum for over 3 h. Killing was due to lysis mediated by both the classical and alternative complement pathways. The bacteria exposed to trospectomycin were enlarged in both diameter and length, but they still grew at rates comparable to those of untreated bacteria. No other visible morphological changes could be directly related to the increase in serum sensitivity. The profile of outer membrane proteins obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis was identical for trospectomycin-treated or untreated bacteria. However, the relative proportion of four major outer membrane proteins varied considerably.
Full text
PDF
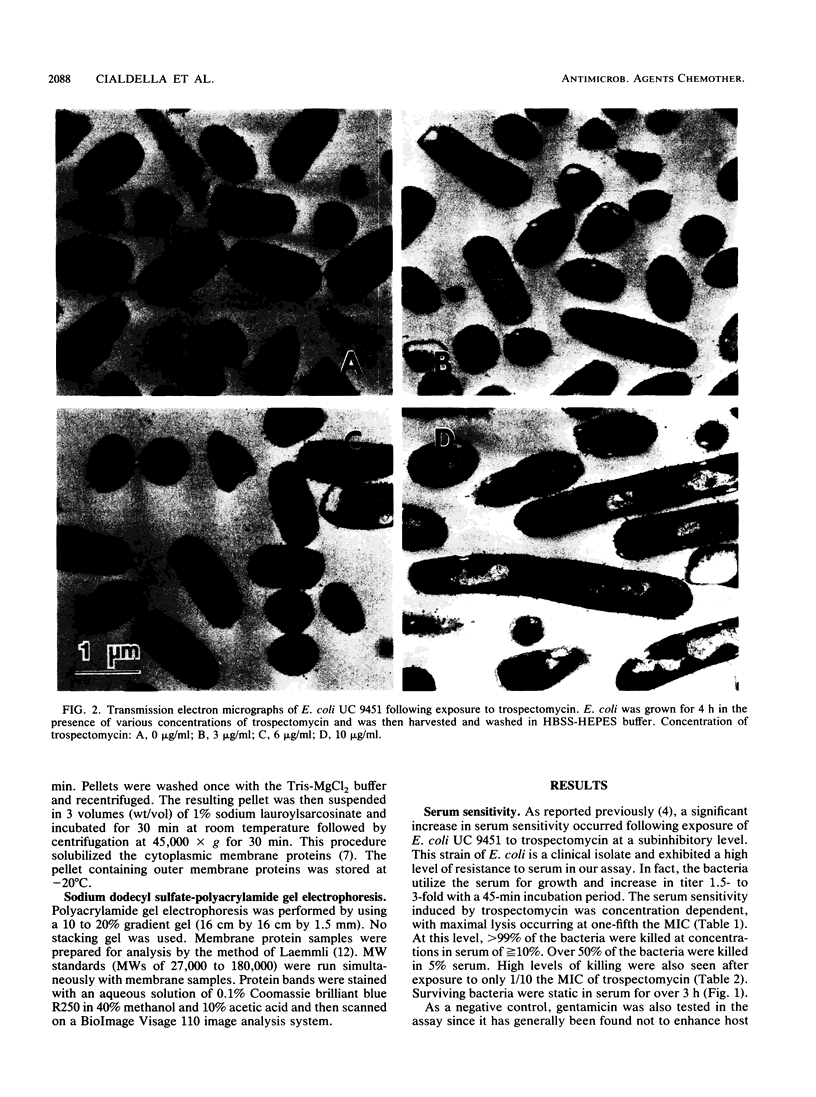
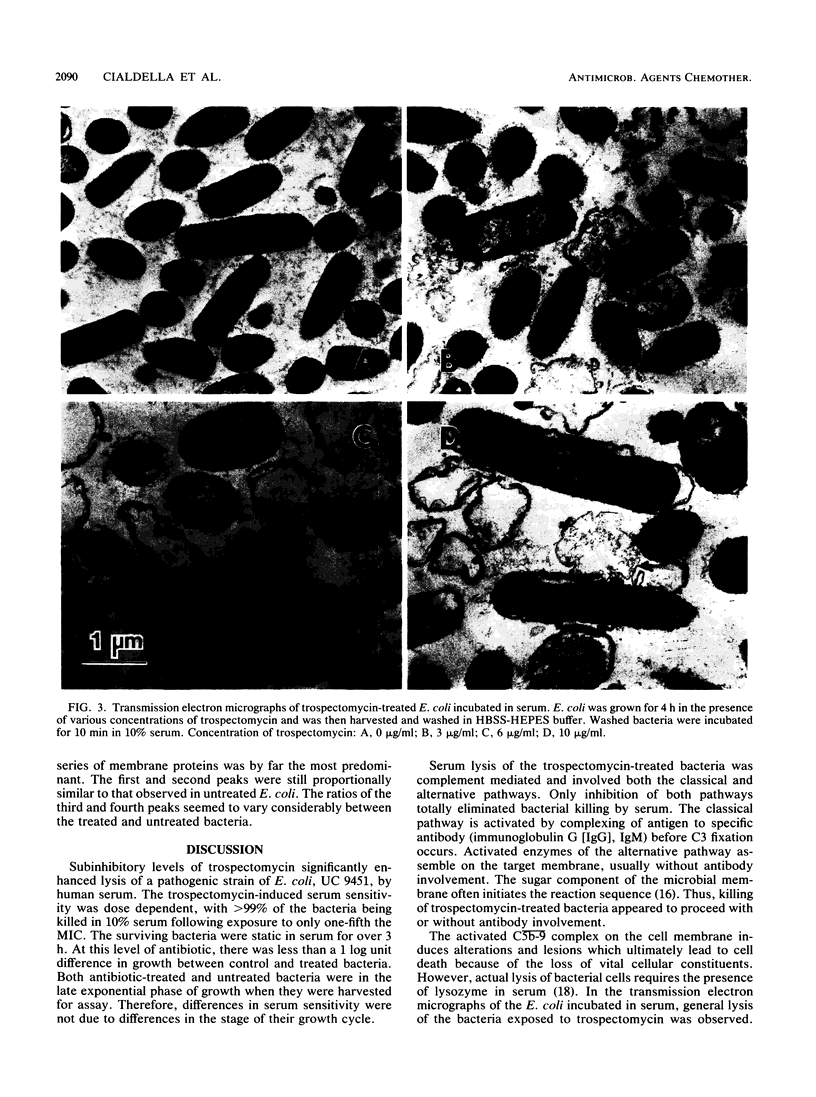
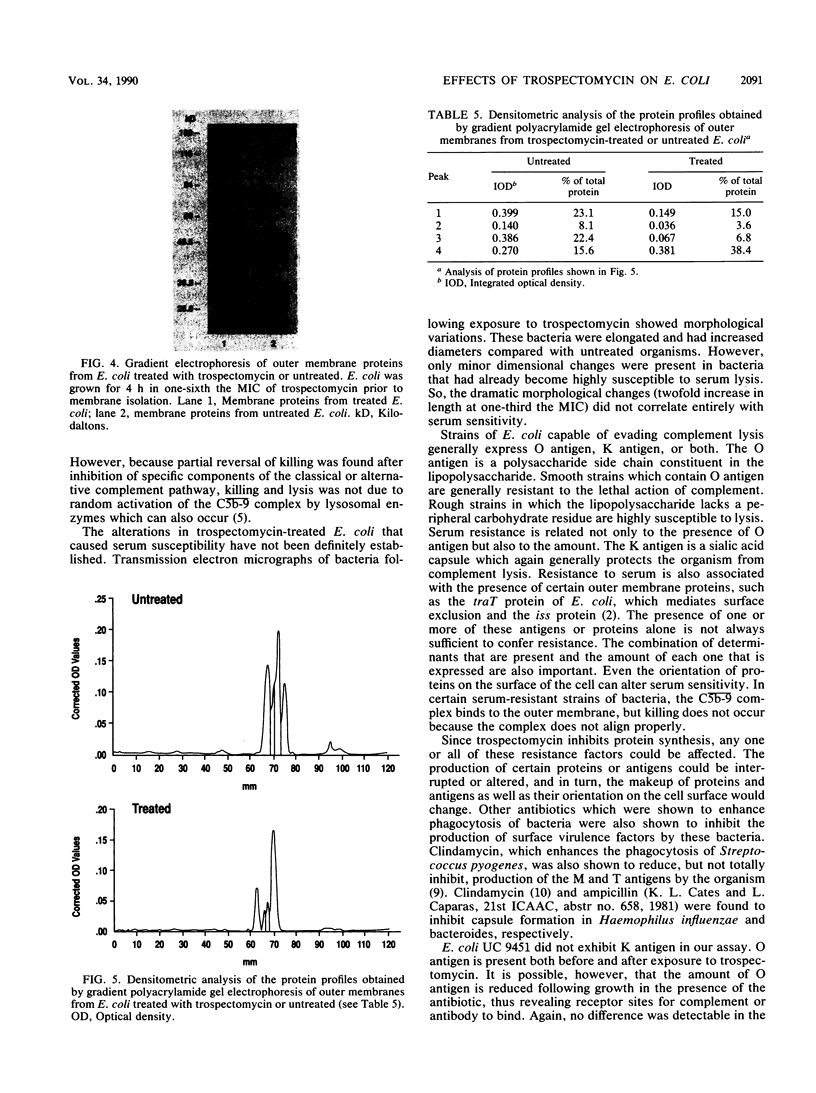

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J., Scott G. K. The effect of purified lipopolysaccharide on the bactericidal reaction of human serum complement. J Gen Microbiol. 1980 Mar;117(1):65–72. doi: 10.1099/00221287-117-1-65. [DOI] [PubMed] [Google Scholar]
- Binns M. M., Mayden J., Levine R. P. Further characterization of complement resistance conferred on Escherichia coli by the plasmid genes traT of R100 and iss of ColV,I-K94. Infect Immun. 1982 Feb;35(2):654–659. doi: 10.1128/iai.35.2.654-659.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdella J. I., Ulrich R. G., Marshall V. P. Augmentation of serum bactericidal activity by paldimycin. J Antibiot (Tokyo) 1988 May;41(5):660–666. doi: 10.7164/antibiotics.41.660. [DOI] [PubMed] [Google Scholar]
- Cialdella J. I., Vavra J. J., Marshall V. P. Susceptibility of bacteria to serum lysis or phagocytosis following growth in subinhibitory levels of lincosaminide or spectinomycin related antibiotics. J Antibiot (Tokyo) 1986 Jul;39(7):978–984. doi: 10.7164/antibiotics.39.978. [DOI] [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsgren A., Mclean R. H., Michael A. F., Quie P. G. Studies of the alternate pathway in chelated serum. J Lab Clin Med. 1975 Jun;85(6):904–912. [PubMed] [Google Scholar]
- Gemmell C. G., Peterson P. K., Schmeling D., Kim Y., Mathews J., Wannamaker L., Quie P. G. Potentiation of opsonization and phagocytosis of Streptococcus pyogenes following growth in the presence of clindamycin. J Clin Invest. 1981 May;67(5):1249–1256. doi: 10.1172/JCI110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopapadakou N. H., Liu F. Y. Penicillin-binding proteins in bacteria. Antimicrob Agents Chemother. 1980 Jul;18(1):148–157. doi: 10.1128/aac.18.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishida M., Mine Y., Nonoyama S., Yokota Y. Effect of antibiotics on the phagocytosis and killing of Pseudomonas aeruginosa by rabbit polymorphonuclear leukocytes. Chemotherapy. 1976;22(3-4):203–210. doi: 10.1159/000221927. [DOI] [PubMed] [Google Scholar]
- Perna P., Andreana A., Dilillo M., Utili R., Ruggiero G. Sensibilita' di germi esposti a concentrazioni subinibenti di antibiotici alla azione del siero e dei fagociti epatici. Boll Soc Ital Biol Sper. 1983 Nov 30;59(11):1683–1686. [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Isturiz R., Molavi A., Metcalf J. A., Malech H. L. Interactions between antibiotics and human neutrophils in the killing of staphylococci. J Clin Invest. 1981 Jan;67(1):247–259. doi: 10.1172/JCI110020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg-Arska M., Porsius J. C., Jaarsma E. Y., Verhoef J. Bactericidal, bacteriolytic and opsonic activity of human serum against Escherichia coli. J Med Microbiol. 1986 Sep;22(2):143–149. doi: 10.1099/00222615-22-2-143. [DOI] [PubMed] [Google Scholar]
- von Graevenitz A. Which bacterial species should be isolated from throat cultures? Eur J Clin Microbiol. 1983 Feb;2(1):1–3. doi: 10.1007/BF02019913. [DOI] [PubMed] [Google Scholar]