Abstract
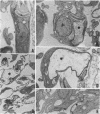
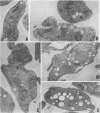
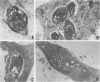
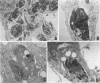
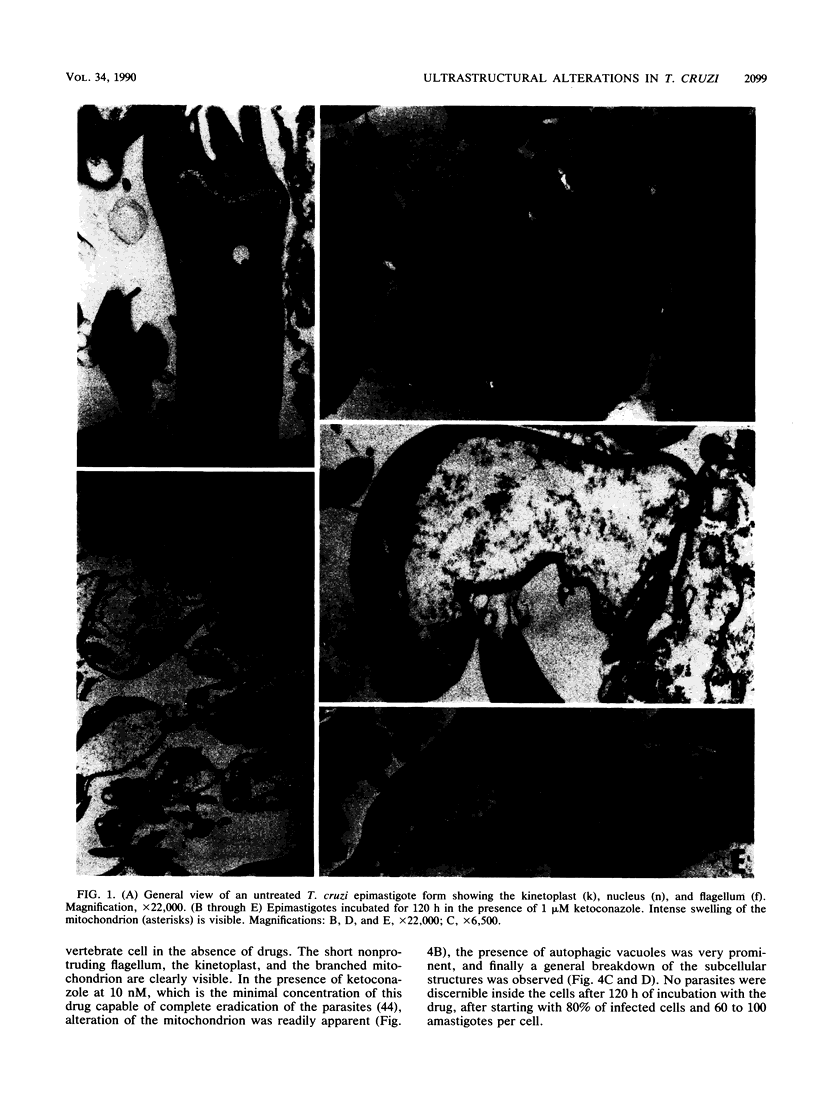
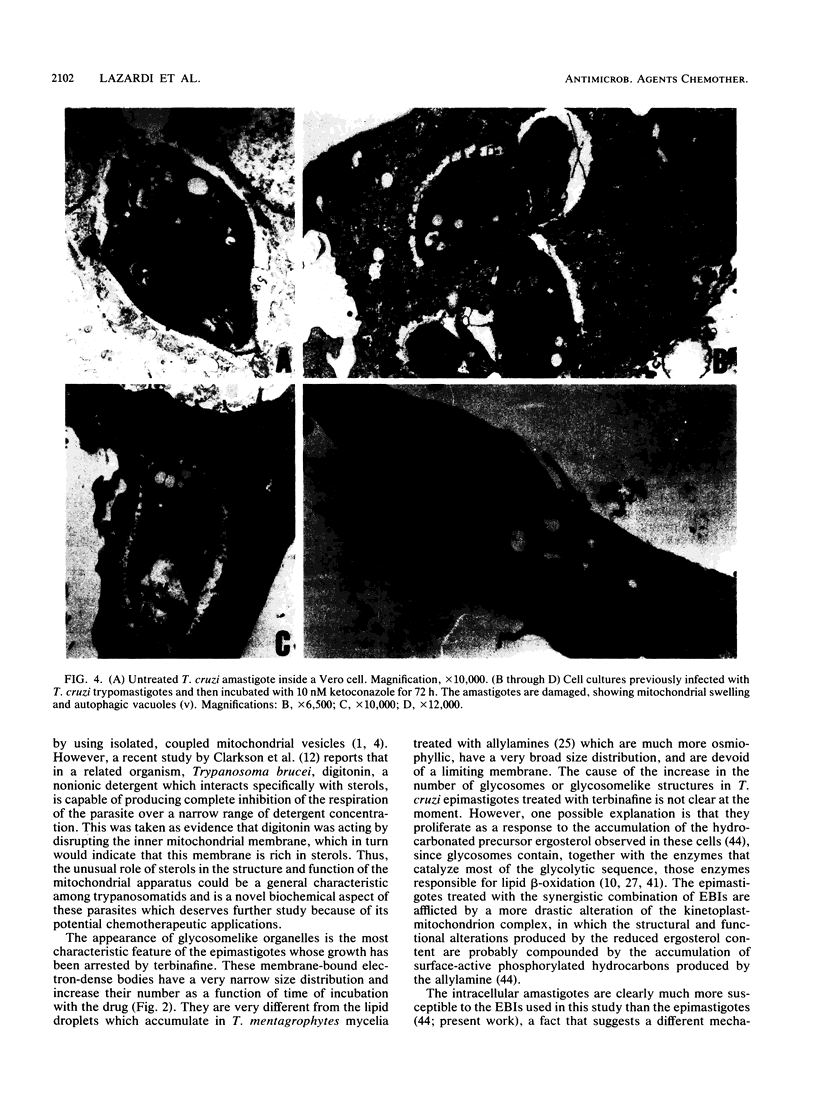
We report the ultrastructural alterations induced during the proliferative stages of Trypanosoma (Schizotrypanum) cruzi, the causative agent of Chagas' disease, by two ergosterol biosynthesis inhibitors, ketoconazole and terbinafine, which had previously been shown to be potent growth inhibitors whose effects are potentiated when used in combination (J. A. Urbina, K. Lazardi, T. Aguirre, M. M. Piras, and R. Piras, Antimicrob. Agents Chemother. 32:1237-1242, 1988). Epimastigotes treated with a low concentration of ketoconazole (1 microM), which blocks ergosterol biosynthesis at the level of C-14 demethylation of lanosterol and induces cell lysis coincident with total ergosterol depletion, showed gross alterations of the kinetoplast-mitochondrion complex, which swelled and lost the organization of its inner membrane and the electron-dense bodies of its matrix. Thus, coincident with the beginning of cell lysis, the kinetoplast-mitochondrion complex occupied greater than 80% of the cell volume, while other subcellular structures such as the nucleus and subpellicular microtubules were not affected. Terbinafine, which blocks ergosterol synthesis in these cells at the level of squalene synthetase and thus leads to almost immediate arrest of growth at concentrations greater than 1 microM, produced proliferation of glycosomelike bodies, binucleated cells (arrest at cytokinesis), and eventually massive vacuolization. When the drugs were combined, the predominant effect was mitochondrial swelling, which was more drastic and took place earlier than that observed in cells treated with ketoconazole alone. In amastigotes proliferating in Vero cells, ketoconazole at the concentration required to eradicate the parasites (10 nM) produced mitochondrial swelling, the appearance of autophagic vacuoles containing partially degraded subcellular material, and finally a general breakdown of the subcellular structures. Terbinafine at 3 microM induced more limited ultrastructural damage to the amastigotes consistent with increased vacuolization of the cells and the appearance of occasional autophagic vacuoles. When the drugs were used in combination, just 1 nM was required for the total eradication of parasites, the ultrastructural effects were more extensive, and cell disintegration occurred earlier than when any of the drugs was used alone at a much higher concentration. No effect of the drugs on the ultrastructure of the host cells were observed at any of the concentrations tested.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Affranchino J. L., De Tarlovsky M. N., Stoppani A. O. Respiratory control in mitochondria from Trypanosoma cruzi. Mol Biochem Parasitol. 1985 Sep;16(3):289–298. doi: 10.1016/0166-6851(85)90071-4. [DOI] [PubMed] [Google Scholar]
- Beach D. H., Goad L. J., Holz G. G., Jr Effects of antimycotic azoles on growth and sterol biosynthesis of Leishmania promastigotes. Mol Biochem Parasitol. 1988 Nov;31(2):149–162. doi: 10.1016/0166-6851(88)90166-1. [DOI] [PubMed] [Google Scholar]
- Beach D. H., Goad L. J., Holz G. G., Jr Effects of ketoconazole on sterol biosynthesis by Trypanosoma cruzi epimastigotes. Biochem Biophys Res Commun. 1986 May 14;136(3):851–856. doi: 10.1016/0006-291x(86)90410-9. [DOI] [PubMed] [Google Scholar]
- Benaim G., Bermudez R., Urbina J. A. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol Biochem Parasitol. 1990 Feb;39(1):61–68. doi: 10.1016/0166-6851(90)90008-a. [DOI] [PubMed] [Google Scholar]
- Berman J. D. Activity of imidazoles against Leishmania tropica in human macrophage cultures. Am J Trop Med Hyg. 1981 May;30(3):566–569. doi: 10.4269/ajtmh.1981.30.566. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Gallalee J. V. In vitro antileishmanial activity of inhibitors of steroid biosynthesis and combinations of antileishmanial agents. J Parasitol. 1987 Jun;73(3):671–673. [PubMed] [Google Scholar]
- Berman J. D., Goad L. J., Beach D. H., Holz G. G., Jr Effects of ketoconazole on sterol biosynthesis by Leishmania mexicana mexicana amastigotes in murine macrophage tumor cells. Mol Biochem Parasitol. 1986 Jul;20(1):85–92. doi: 10.1016/0166-6851(86)90145-3. [DOI] [PubMed] [Google Scholar]
- Berman J. D., Holz G. G., Jr, Beach D. H. Effects of ketoconazole on growth and sterol biosynthesis of Leishmania mexicana promastigotes in culture. Mol Biochem Parasitol. 1984 May;12(1):1–13. doi: 10.1016/0166-6851(84)90039-2. [DOI] [PubMed] [Google Scholar]
- Bloch K. E. Sterol structure and membrane function. CRC Crit Rev Biochem. 1983;14(1):47–92. doi: 10.3109/10409238309102790. [DOI] [PubMed] [Google Scholar]
- Cannata J. J., Valle E., Docampo R., Cazzulo J. J. Subcellular localization of phosphoenolpyruvate carboxykinase in the trypanosomatids Trypanosoma cruzi and Crithidia fasciculata. Mol Biochem Parasitol. 1982 Sep;6(3):151–160. doi: 10.1016/0166-6851(82)90074-3. [DOI] [PubMed] [Google Scholar]
- Cannon R. D., Kerridge D. Correlation between the sterol composition of membranes and morphology in Candida albicans. J Med Vet Mycol. 1988 Feb;26(1):57–65. doi: 10.1080/02681218880000071. [DOI] [PubMed] [Google Scholar]
- Clarkson A. B., Jr, Bienen E. J., Pollakis G., Grady R. W. Respiration of bloodstream forms of the parasite Trypanosoma brucei brucei is dependent on a plant-like alternative oxidase. J Biol Chem. 1989 Oct 25;264(30):17770–17776. [PubMed] [Google Scholar]
- Dahl C., Biemann H. P., Dahl J. A protein kinase antigenically related to pp60v-src possibly involved in yeast cell cycle control: positive in vivo regulation by sterol. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4012–4016. doi: 10.1073/pnas.84.12.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docampo R., Moreno S. N., Turrens J. F., Katzin A. M., Gonzalez-Cappa S. M., Stoppani A. O. Biochemical and ultrastructural alterations produced by miconazole and econazole in Trypanosoma cruzi. Mol Biochem Parasitol. 1981 Jul;3(3):169–180. doi: 10.1016/0166-6851(81)90047-5. [DOI] [PubMed] [Google Scholar]
- Georgopoulos A., Petranyi G., Mieth H., Drews J. In vitro activity of naftifine, a new antifungal agent. Antimicrob Agents Chemother. 1981 Mar;19(3):386–389. doi: 10.1128/aac.19.3.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goad L. J., Berens R. L., Marr J. J., Beach D. H., Holz G. G., Jr The activity of ketoconazole and other azoles against Trypanosoma cruzi: biochemistry and chemotherapeutic action in vitro. Mol Biochem Parasitol. 1989 Jan 15;32(2-3):179–189. doi: 10.1016/0166-6851(89)90069-8. [DOI] [PubMed] [Google Scholar]
- Goad L. J., Holz G. G., Jr, Beach D. H. Sterols of ketoconazole-inhibited Leishmania mexicana mexicana promastigotes. Mol Biochem Parasitol. 1985 Jun;15(3):257–279. doi: 10.1016/0166-6851(85)90089-1. [DOI] [PubMed] [Google Scholar]
- Hart D. T., Lauwers W. J., Willemsens G., Vanden Bossche H., Opperdoes F. R. Perturbation of sterol biosynthesis by itraconazole and ketoconazole in Leishmania mexicana mexicana infected macrophages. Mol Biochem Parasitol. 1989 Mar 1;33(2):123–134. doi: 10.1016/0166-6851(89)90026-1. [DOI] [PubMed] [Google Scholar]
- Heeres J., Backx L. J., Mostmans J. H., Van Cutsem J. Antimycotic imidazoles. part 4. Synthesis and antifungal activity of ketoconazole, a new potent orally active broad-spectrum antifungal agent. J Med Chem. 1979 Aug;22(8):1003–1005. doi: 10.1021/jm00194a023. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Berman J. D., Riordan G. P., Lee L. S. Fine-structural alterations in Leishmania tropica within human macrophages exposed to antileishmanial drugs in vitro. J Protozool. 1983 Aug;30(3):555–561. doi: 10.1111/j.1550-7408.1983.tb01421.x. [DOI] [PubMed] [Google Scholar]
- Larralde G., Vivas J., Urbina J. A. Concentration and time dependence of the effects of ketoconazole on growth and sterol synthesis by Trypanosoma (Schizotrypanum) cruzi epimastigotes. Acta Cient Venez. 1988;39(2):140–146. [PubMed] [Google Scholar]
- McCabe R. E., Remington J. S., Araujo F. G. In vitro and in vivo effects of itraconazole against Trypanosoma cruzi. Am J Trop Med Hyg. 1986 Mar;35(2):280–284. doi: 10.4269/ajtmh.1986.35.280. [DOI] [PubMed] [Google Scholar]
- McCabe R. E., Remington J. S., Araujo F. G. Ketoconazole inhibition of intracellular multiplication of Trypanosoma cruzi and protection of mice against lethal infection with the organism. J Infect Dis. 1984 Oct;150(4):594–601. doi: 10.1093/infdis/150.4.594. [DOI] [PubMed] [Google Scholar]
- Meingassner J. G., Sleytr U., Petranyi G. Morphological changes induced by Naftifine, a new antifungal agent, in Trichophyton mentagrophytes. J Invest Dermatol. 1981 Dec;77(6):444–451. doi: 10.1111/1523-1747.ep12495814. [DOI] [PubMed] [Google Scholar]
- Nes W. R., Sekula B. C., Nes W. D., Adler J. H. The functional importance of structural features of ergosterol in yeast. J Biol Chem. 1978 Sep 10;253(17):6218–6225. [PubMed] [Google Scholar]
- Opperdoes F. R. Compartmentation of carbohydrate metabolism in trypanosomes. Annu Rev Microbiol. 1987;41:127–151. doi: 10.1146/annurev.mi.41.100187.001015. [DOI] [PubMed] [Google Scholar]
- Petranyi G., Georgopoulos A., Mieth H. In vivo antimycotic activity of naftifine. Antimicrob Agents Chemother. 1981 Mar;19(3):390–392. doi: 10.1128/aac.19.3.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petranyi G., Meingassner J. G., Mieth H. Activity of terbinafine in experimental fungal infections of laboratory animals. Antimicrob Agents Chemother. 1987 Oct;31(10):1558–1561. doi: 10.1128/aac.31.10.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petranyi G., Meingassner J. G., Mieth H. Antifungal activity of the allylamine derivative terbinafine in vitro. Antimicrob Agents Chemother. 1987 Sep;31(9):1365–1368. doi: 10.1128/aac.31.9.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petranyi G., Ryder N. S., Stütz A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science. 1984 Jun 15;224(4654):1239–1241. doi: 10.1126/science.6547247. [DOI] [PubMed] [Google Scholar]
- Pinto W. J., Nes W. R. Stereochemical specificity for sterols in Saccharomyces cerevisiae. J Biol Chem. 1983 Apr 10;258(7):4472–4476. [PubMed] [Google Scholar]
- Ryder N. S., Dupont M. C. Inhibition of squalene epoxidase by allylamine antimycotic compounds. A comparative study of the fungal and mammalian enzymes. Biochem J. 1985 Sep 15;230(3):765–770. doi: 10.1042/bj2300765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder N. S. Effect of allylamine antimycotic agents on fungal sterol biosynthesis measured by sterol side-chain methylation. J Gen Microbiol. 1985 Jul;131(7):1595–1602. doi: 10.1099/00221287-131-7-1595. [DOI] [PubMed] [Google Scholar]
- Ryder N. S. Mechanism of action and biochemical selectivity of allylamine antimycotic agents. Ann N Y Acad Sci. 1988;544:208–220. doi: 10.1111/j.1749-6632.1988.tb40405.x. [DOI] [PubMed] [Google Scholar]
- Ryder N. S., Seidl G., Troke P. F. Effect of the antimycotic drug naftifine on growth of and sterol biosynthesis in Candida albicans. Antimicrob Agents Chemother. 1984 Apr;25(4):483–487. doi: 10.1128/aac.25.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryder N. S. Specific inhibition of fungal sterol biosynthesis by SF 86-327, a new allylamine antimycotic agent. Antimicrob Agents Chemother. 1985 Feb;27(2):252–256. doi: 10.1128/aac.27.2.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Heterogeneity of action of mechanisms among antimycotic imidazoles. Antimicrob Agents Chemother. 1981 Jul;20(1):71–74. doi: 10.1128/aac.20.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sud I. J., Feingold D. S. Mechanisms of action of the antimycotic imidazoles. J Invest Dermatol. 1981 Jun;76(6):438–441. doi: 10.1111/1523-1747.ep12521036. [DOI] [PubMed] [Google Scholar]
- Taylor M. B., Berghausen H., Heyworth P., Messenger N., Rees L. J., Gutteridge W. E. Subcellular localization of some glycolytic enzymes in parasitic flagellated protozoa. Int J Biochem. 1980;11(2):117–120. doi: 10.1016/0020-711x(80)90243-8. [DOI] [PubMed] [Google Scholar]
- Troke P. F., Marriott M. S., Richardson K., Tarbit M. H. In vitro potency and in vivo activity of azoles. Ann N Y Acad Sci. 1988;544:284–293. doi: 10.1111/j.1749-6632.1988.tb40414.x. [DOI] [PubMed] [Google Scholar]
- Turrens J. F., Boveris A., Gros E. G., Stoppani A. O. Distribución subcelular de ergosterol y esteroles 5,7- diénicos en Trypanosoma cruzi. Medicina (B Aires) 1980;40 (Suppl 1):137–144. [PubMed] [Google Scholar]
- Urbina J. A., Vivas J., Ramos H., Larralde G., Aguilar Z., Avilán L. Alteration of lipid order profile and permeability of plasma membranes from Trypanosoma cruzi epimastigotes grown in the presence of ketoconazole. Mol Biochem Parasitol. 1988 Aug;30(2):185–195. doi: 10.1016/0166-6851(88)90111-9. [DOI] [PubMed] [Google Scholar]
- Urcuyo F. G., Zaias N. Oral ketoconazole in the treatment of leishmaniasis. Int J Dermatol. 1982 Sep;21(7):414–416. doi: 10.1111/j.1365-4362.1982.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Van den Bossche H., Willemsens G., Cools W., Cornelissen F., Lauwers W. F., van Cutsem J. M. In vitro and in vivo effects of the antimycotic drug ketoconazole on sterol synthesis. Antimicrob Agents Chemother. 1980 Jun;17(6):922–928. doi: 10.1128/aac.17.6.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Bossche H., Marichal P., Gorrens J., Geerts H., Janssen P. A. Mode of action studies. Basis for the search of new antifungal drugs. Ann N Y Acad Sci. 1988;544:191–207. doi: 10.1111/j.1749-6632.1988.tb40404.x. [DOI] [PubMed] [Google Scholar]
- Weinrauch L., Livshin R., El-On J. Cutaneous leishmaniasis: treatment with ketoconazole. Cutis. 1983 Sep;32(3):288-9, 294. [PubMed] [Google Scholar]
- de Souza W. Cell biology of Trypanosoma cruzi. Int Rev Cytol. 1984;86:197–283. doi: 10.1016/s0074-7696(08)60180-1. [DOI] [PubMed] [Google Scholar]
- van den Bossche H., Willemsens G., Cools W., Lauwers W. F., Le Jeune L. Biochemical effects of miconazole on fungi. II. Inhibition of ergosterol biosynthesis in Candida albicans. Chem Biol Interact. 1978 Apr;21(1):59–78. doi: 10.1016/0009-2797(78)90068-6. [DOI] [PubMed] [Google Scholar]