Abstract
Klebsiella pneumoniae isolates from 11 patients at the Miriam Hospital were identified as resistant to cefoxitin and ceftibuten as well as to aztreonam, cefotaxime, and ceftazidime. Resistance could be transferred by conjugation or transformation with plasmid DNA into Escherichia coli and was due to the production of a beta-lactamase with an isoelectric point of 8.4 named MIR-1. In E. coli, MIR-1 conferred resistance to aztreonam, cefotaxime, ceftazidime, ceftibuten, ceftriaxone, and such alpha-methoxy beta-lactams as cefmetazole, cefotetan, cefoxitin, and moxalactam. In vitro, MIR-1 hydrolyzed cephalothin and cephaloridine much more rapidly than it did penicillin G, ampicillin, or carbenicillin. Cefotaxime was hydrolyzed at 10% the rate of cephaloridine. Cefoxitin inactivation could only be detected by a microbiological test. The inhibition profile of MIR-1 was similar to that of chromosomally mediated class I beta-lactamases. Potassium clavulanate had little effect on cefoxitin or cefibuten resistance and was a poor inhibitor of MIR-1 activity. Cefoxitin or imipenem did not induce MIR-1. The gene determining MIR-1 was cloned on a 1.4-kb AccI-PstI fragment. Under stringent conditions, probes for TEM-1 and SHV-1 genes and the E. coli ampC gene failed to hybridize with the MIR-1 gene. However, a provisional sequence of 150 bp of the MIR-1 gene proved to be 90% identical to the sequence of ampC from Enterobacter cloacae but only 71% identical to that of E. coli, thus explaining the lack of hybridization to the E. coli ampC probe. Plasmid profiles of the 11 K. pneumoniae clinical isolates were not identical, but each contained a plasmid from 40 to 60 kb that hybridized with the cloned MIR-1 gene. Both transfer-proficient and transfer-deficient MIR-1 plasmids belonged to the N incompatibility group. Thus, the resistance of these K. pneumoniae strains was the result of plasmid acquisition of a class I beta-lactamase, a new resistance determinant that expands the kinds of beta-lactam resistance capable of spread by plasmid dissemination among clinical isolates.
Full text
PDF

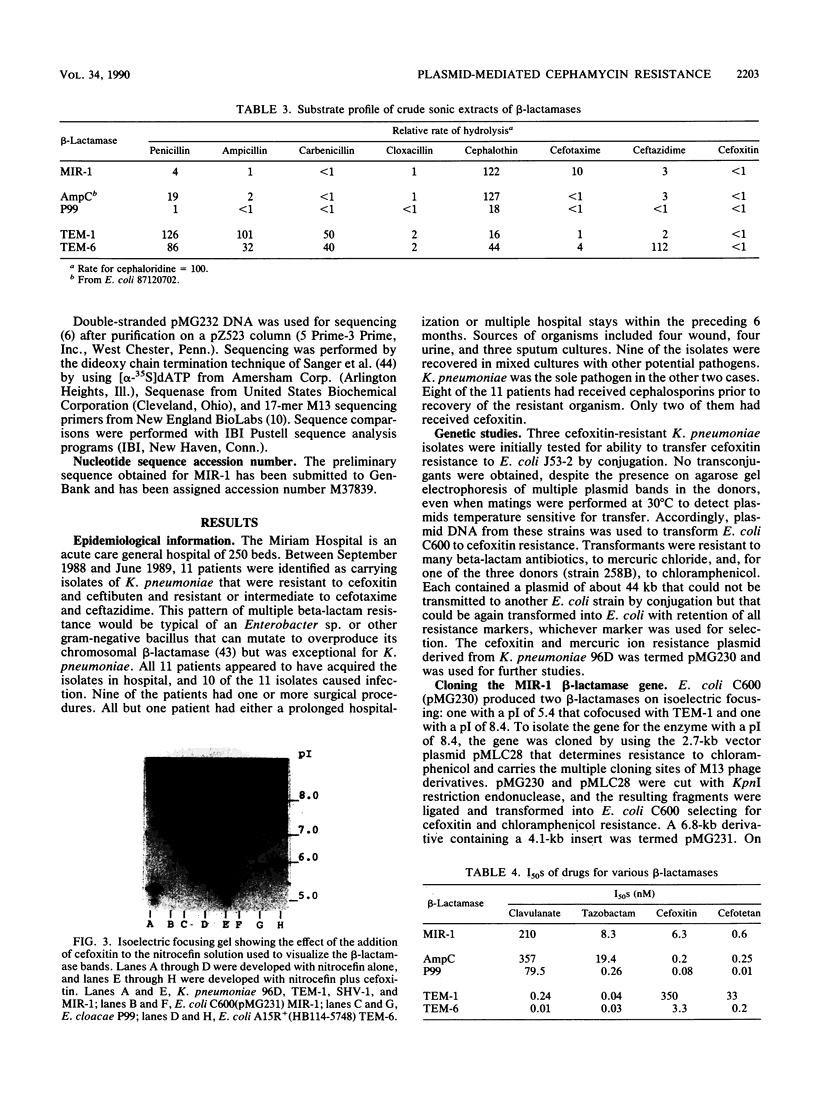
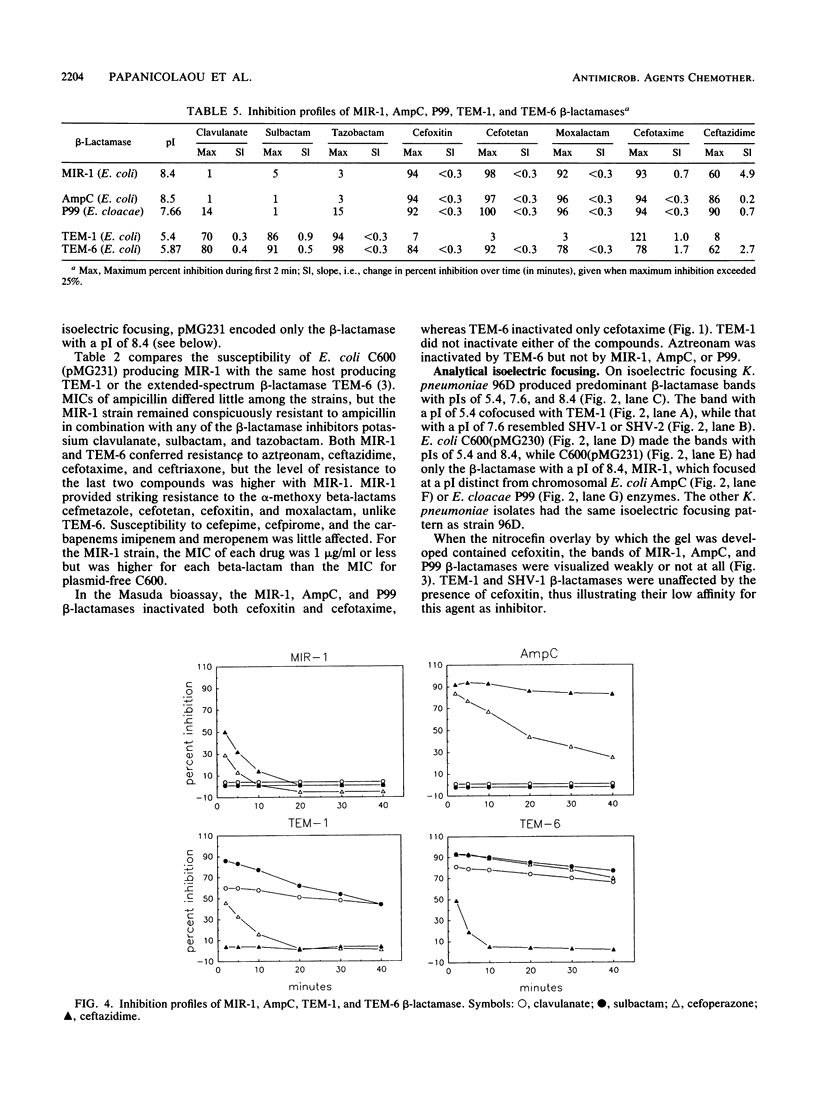
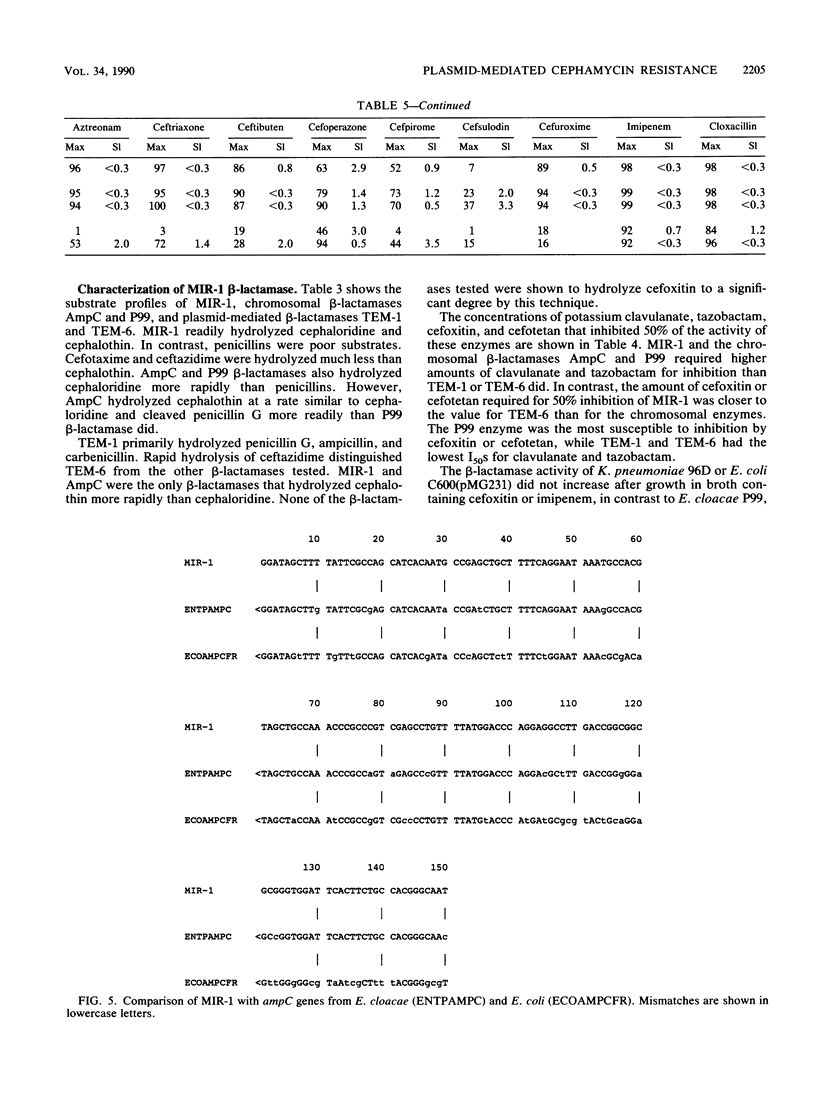

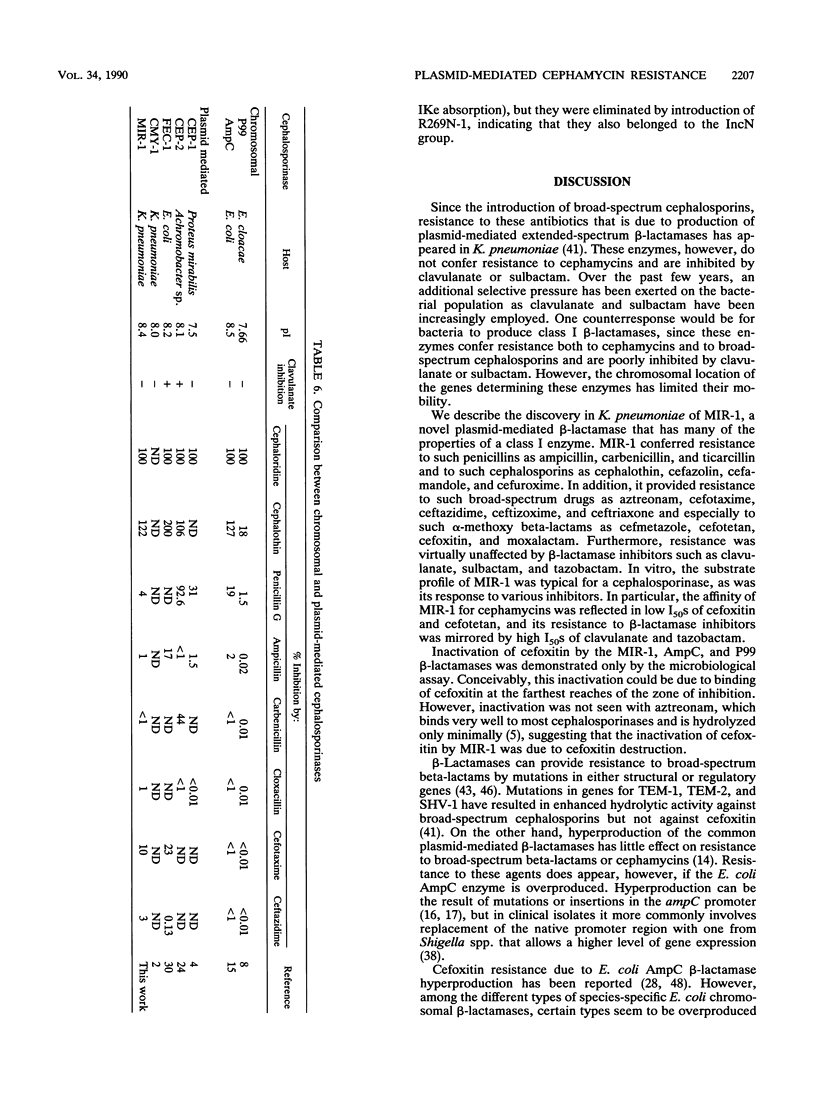


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa Y., Ohta M., Kido N., Fujii Y., Komatsu T., Kato N. Close evolutionary relationship between the chromosomally encoded beta-lactamase gene of Klebsiella pneumoniae and the TEM beta-lactamase gene mediated by R plasmids. FEBS Lett. 1986 Oct 20;207(1):69–74. doi: 10.1016/0014-5793(86)80014-x. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Chong Y., Schweighart S. Extended broad spectrum beta-lactamase in Klebsiella pneumoniae including resistance to cephamycins. Infection. 1989 Sep-Oct;17(5):316–321. doi: 10.1007/BF01650718. [DOI] [PubMed] [Google Scholar]
- Bauernfeind A., Hörl G. Novel R-factor borne beta-lactamase of Escherichia coli confering resistance to cephalosporins. Infection. 1987 Jul-Aug;15(4):257–259. doi: 10.1007/BF01644127. [DOI] [PubMed] [Google Scholar]
- Bobrowski M. M., Matthew M., Barth P. T., Datta N., Grinter N. J., Jacob A. E., Kontomichalou P., Dale J. W., Smith J. T. Plasmid-determined beta-lactamase indistinguishable from the chromosomal beta-lactamase of Escherichia coli. J Bacteriol. 1976 Jan;125(1):149–157. doi: 10.1128/jb.125.1.149-157.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush K., Tanaka S. K., Bonner D. P., Sykes R. B. Resistance caused by decreased penetration of beta-lactam antibiotics into Enterobacter cloacae. Antimicrob Agents Chemother. 1985 Apr;27(4):555–560. doi: 10.1128/aac.27.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Coetzee J. N., Datta N., Hedges R. W. R factors from Proteus rettgeri. J Gen Microbiol. 1972 Oct;72(3):543–552. doi: 10.1099/00221287-72-3-543. [DOI] [PubMed] [Google Scholar]
- Galleni M., Lindberg F., Normark S., Cole S., Honore N., Joris B., Frere J. M. Sequence and comparative analysis of three Enterobacter cloacae ampC beta-lactamase genes and their products. Biochem J. 1988 Mar 15;250(3):753–760. doi: 10.1042/bj2500753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Goering R. V. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. doi: 10.1093/infdis/146.1.34. [DOI] [PubMed] [Google Scholar]
- Huovinen P., Huovinen S., Jacoby G. A. Sequence of PSE-2 beta-lactamase. Antimicrob Agents Chemother. 1988 Jan;32(1):134–136. doi: 10.1128/aac.32.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huovinen S., Huovinén P., Jacoby G. A. Detection of plasmid-mediated beta-lactamases with DNA probes. Antimicrob Agents Chemother. 1988 Feb;32(2):175–179. doi: 10.1128/aac.32.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Carreras I. Activities of beta-lactam antibiotics against Escherichia coli strains producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1990 May;34(5):858–862. doi: 10.1128/aac.34.5.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby G. A., Sutton L. beta-Lactamases and beta-lactam resistance in Escherichia coli. Antimicrob Agents Chemother. 1985 Nov;28(5):703–705. doi: 10.1128/aac.28.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Grundström T., Normark S. Sequence elements determining ampC promoter strength in E. coli. EMBO J. 1982;1(7):875–881. doi: 10.1002/j.1460-2075.1982.tb01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Grundström T. ampC cephalosporinase of Escherichia coli K-12 has a different evolutionary origin from that of beta-lactamases of the penicillinase type. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4897–4901. doi: 10.1073/pnas.78.8.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaurin B., Normark S. Insertion of IS2 creates a novel ampC promoter in Escherichia coli. Cell. 1983 Mar;32(3):809–816. doi: 10.1016/0092-8674(83)90067-3. [DOI] [PubMed] [Google Scholar]
- Khatoon H., Iyer R. V., Iyer V. N. A new filamentous bacteriophage with sex-factor specificity. Virology. 1972 Apr;48(1):145–155. doi: 10.1016/0042-6822(72)90122-5. [DOI] [PubMed] [Google Scholar]
- Kliebe C., Nies B. A., Meyer J. F., Tolxdorff-Neutzling R. M., Wiedemann B. Evolution of plasmid-coded resistance to broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1985 Aug;28(2):302–307. doi: 10.1128/aac.28.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knothe H., Shah P., Krcmery V., Antal M., Mitsuhashi S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 1983 Nov-Dec;11(6):315–317. doi: 10.1007/BF01641355. [DOI] [PubMed] [Google Scholar]
- Korfmann G., Kliebe C., Wiedemann B. Beta-lactam antibiotics and selection of resistance: speculation on the evolution of R-plasmids. J Antimicrob Chemother. 1986 Oct;18 (Suppl 100):113–121. doi: 10.1093/jac/18.supplement_c.113. [DOI] [PubMed] [Google Scholar]
- Labia R., Andrillon J., Le Goffic F. Computerized microacidimetric determination of beta lactamase Michaelis-Menten constants. FEBS Lett. 1973 Jun 15;33(1):42–44. doi: 10.1016/0014-5793(73)80154-1. [DOI] [PubMed] [Google Scholar]
- Lederberg E. M., Cohen S. N. Transformation of Salmonella typhimurium by plasmid deoxyribonucleic acid. J Bacteriol. 1974 Sep;119(3):1072–1074. doi: 10.1128/jb.119.3.1072-1074.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque R. C., Medeiros A. A., Jacoby G. A. Molecular cloning and DNA homology of plasmid-mediated beta-lactamase genes. Mol Gen Genet. 1987 Feb;206(2):252–258. doi: 10.1007/BF00333581. [DOI] [PubMed] [Google Scholar]
- Levesque R., Roy P. H., Letarte R., Pechère J. C. A plasmid-mediated cephalosporinase from Achromobacter species. J Infect Dis. 1982 May;145(5):753–761. doi: 10.1093/infdis/145.2.753. [DOI] [PubMed] [Google Scholar]
- Levinson A., Silver D., Seed B. Minimal size plasmids containing an M13 origin for production of single-strand transducing particles. J Mol Appl Genet. 1984;2(6):507–517. [PubMed] [Google Scholar]
- Macrina F. L., Kopecko D. J., Jones K. R., Ayers D. J., McCowen S. M. A multiple plasmid-containing Escherichia coli strain: convenient source of size reference plasmid molecules. Plasmid. 1978 Jun;1(3):417–420. doi: 10.1016/0147-619x(78)90056-2. [DOI] [PubMed] [Google Scholar]
- Marre R., Aleksic S. Beta-lactamase types and beta-lactam resistance of Escherichia coli strains with chromosomally mediated ampicillin resistance. Eur J Clin Microbiol Infect Dis. 1990 Jan;9(1):44–46. doi: 10.1007/BF01969534. [DOI] [PubMed] [Google Scholar]
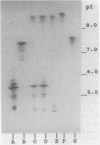
- Masuda G., Tomioka S., Hasegawa M. Detection of beta-lactamase production by gram-negative bacteria. J Antibiot (Tokyo) 1976 Jun;29(6):662–664. doi: 10.7164/antibiotics.29.662. [DOI] [PubMed] [Google Scholar]
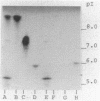
- Mathew A., Harris A. M., Marshall M. J., Ross G. W. The use of analytical isoelectric focusing for detection and identification of beta-lactamases. J Gen Microbiol. 1975 May;88(1):169–178. doi: 10.1099/00221287-88-1-169. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y., Ikeda F., Kamimura T., Yokota Y., Mine Y. Novel plasmid-mediated beta-lactamase from Escherichia coli that inactivates oxyimino-cephalosporins. Antimicrob Agents Chemother. 1988 Aug;32(8):1243–1246. doi: 10.1128/aac.32.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthew M., Hedges R. W., Smith J. T. Types of beta-lactamase determined by plasmids in gram-negative bacteria. J Bacteriol. 1979 Jun;138(3):657–662. doi: 10.1128/jb.138.3.657-662.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros A. A., Cohenford M., Jacoby G. A. Five novel plasmid-determined beta-lactamases. Antimicrob Agents Chemother. 1985 May;27(5):715–719. doi: 10.1128/aac.27.5.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercier J., Levesque R. C. Cloning of SHV-2, OHIO-1, and OXA-6 beta-lactamases and cloning and sequencing of SHV-1 beta-lactamase. Antimicrob Agents Chemother. 1990 Aug;34(8):1577–1583. doi: 10.1128/aac.34.8.1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan C. H., Morris A., Kirby S. M., Shingler A. H. Novel method for detection of beta-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972 Apr;1(4):283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson O., Bergström S., Lindberg F. P., Normark S. ampC beta-lactamase hyperproduction in Escherichia coli: natural ampicillin resistance generated by horizontal chromosomal DNA transfer from Shigella. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7556–7560. doi: 10.1073/pnas.80.24.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papanicolaou G. A., Medeiros A. A. Discrimination of extended-spectrum beta-lactamases by a novel nitrocefin competition assay. Antimicrob Agents Chemother. 1990 Nov;34(11):2184–2192. doi: 10.1128/aac.34.11.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit A., Sirot D. L., Chanal C. M., Sirot J. L., Labia R., Gerbaud G., Cluzel R. A. Novel plasmid-mediated beta-lactamase in clinical isolates of Klebsiella pneumoniae more resistant to ceftazidime than to other broad-spectrum cephalosporins. Antimicrob Agents Chemother. 1988 May;32(5):626–630. doi: 10.1128/aac.32.5.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A. M., Paul G. C., Jacoby G. A. Properties of PSE-2 beta-lactamase and genetic basis for its production in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1983 Sep;24(3):362–369. doi: 10.1128/aac.24.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippon A., Labia R., Jacoby G. Extended-spectrum beta-lactamases. Antimicrob Agents Chemother. 1989 Aug;33(8):1131–1136. doi: 10.1128/aac.33.8.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C. Chromosomal cephalosporinases responsible for multiple resistance to newer beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:573–593. doi: 10.1146/annurev.mi.41.100187.003041. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirot D., Sirot J., Labia R., Morand A., Courvalin P., Darfeuille-Michaud A., Perroux R., Cluzel R. Transferable resistance to third-generation cephalosporins in clinical isolates of Klebsiella pneumoniae: identification of CTX-1, a novel beta-lactamase. J Antimicrob Chemother. 1987 Sep;20(3):323–334. doi: 10.1093/jac/20.3.323. [DOI] [PubMed] [Google Scholar]
- Sougakoff W., Goussard S., Gerbaud G., Courvalin P. Plasmid-mediated resistance to third-generation cephalosporins caused by point mutations in TEM-type penicillinase genes. Rev Infect Dis. 1988 Jul-Aug;10(4):879–884. doi: 10.1093/clinids/10.4.879. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Sawai T., Ando T., Yamagishi S. Cefoxitin resistance by a chromosomal cephalosporinase in Escherichia coli. J Antibiot (Tokyo) 1980 Sep;33(9):1037–1042. doi: 10.7164/antibiotics.33.1037. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984 Oct;20(4):608–613. doi: 10.1128/jcm.20.4.608-613.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecoli C., Prevost F. E., Ververis J. J., Medeiros A. A., O'Leary G. P., Jr Comparison of polyacrylamide and agarose gel thin-layer isoelectric focusing for the characterization of beta-lactamases. Antimicrob Agents Chemother. 1983 Aug;24(2):186–189. doi: 10.1128/aac.24.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]