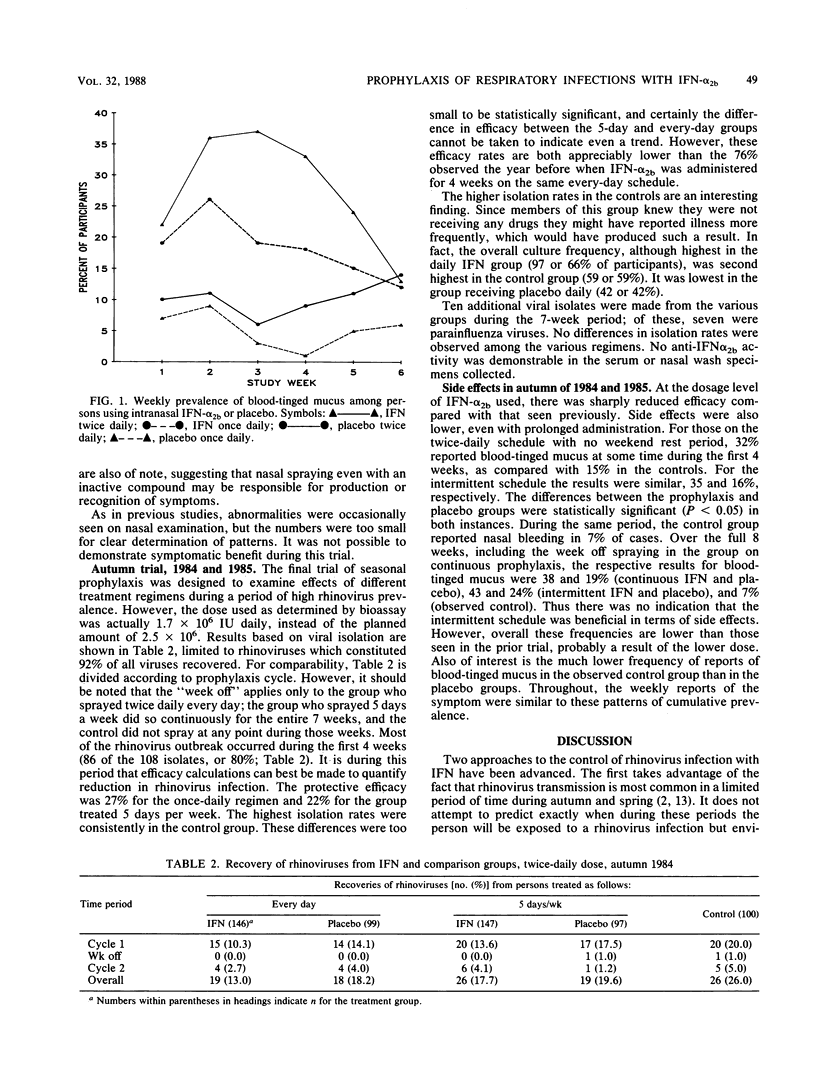
Abstract
Recombinant alpha-2b interferon was evaluated in two controlled trials, each lasting for 2 months or more, with different dose levels and schedules of administration. The first study was conducted during a period of transmission of type A (H1N1) and type B influenza. At 2.5 x 10(6) IU per day, no effect on influenza infection could be detected, but there appeared to be an effect on rhinovirus isolation. During the subsequent autumn 1.7 x 10(6) IU per day was found to have only a minimal effect on rhinovirus infection (efficacy from 22 to 27%). Under similar circumstances the preceding year, but with a daily dose of 3.0 x 10(6) IU, efficacy had been 76%. Since there was no evidence of change in rhinovirus strains circulating or their interferon susceptibility, this represented a dose-response relationship. It was possible to evaluate side effects in the 1,200 individuals involved. A lower dose was associated with lower frequency of symptoms of blood-tinged mucus. Persons using a placebo spray had a higher frequency of this side effect than an observed control. Using the spray 5 days a week was no less likely to produce symptoms than everyday use. Once-daily use was less likely to produce side effects than twice-daily use. There was no indication of sensitization when interferon was used for two separate periods of 4 weeks.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dolin R., Reichman R. C., Madore H. P., Maynard R., Linton P. N., Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982 Sep 2;307(10):580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- Douglas R. M., Albrecht J. K., Miles H. B., Moore B. W., Read R., Worswick D. A., Woodward A. J. Intranasal interferon-alpha 2 prophylaxis of natural respiratory virus infection. J Infect Dis. 1985 Apr;151(4):731–736. doi: 10.1093/infdis/151.4.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R. M., Moore B. W., Miles H. B., Davies L. M., Graham N. M., Ryan P., Worswick D. A., Albrecht J. K. Prophylactic efficacy of intranasal alpha 2-interferon against rhinovirus infections in the family setting. N Engl J Med. 1986 Jan 9;314(2):65–70. doi: 10.1056/NEJM198601093140201. [DOI] [PubMed] [Google Scholar]
- Farr B. M., Gwaltney J. M., Jr, Adams K. F., Hayden F. G. Intranasal interferon-alpha 2 for prevention of natural rhinovirus colds. Antimicrob Agents Chemother. 1984 Jul;26(1):31–34. doi: 10.1128/aac.26.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden F. G., Albrecht J. K., Kaiser D. L., Gwaltney J. M., Jr Prevention of natural colds by contact prophylaxis with intranasal alpha 2-interferon. N Engl J Med. 1986 Jan 9;314(2):71–75. doi: 10.1056/NEJM198601093140202. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Gwaltney J. M., Jr Intranasal interferon alpha 2 for prevention of rhinovirus infection and illness. J Infect Dis. 1983 Sep;148(3):543–550. doi: 10.1093/infdis/148.3.543. [DOI] [PubMed] [Google Scholar]
- Hayden F. G., Gwaltney J. M., Jr, Johns M. E. Prophylactic efficacy and tolerance of low-dose intranasal interferon-alpha 2 in natural respiratory viral infections. Antiviral Res. 1985 Apr;5(2):111–116. doi: 10.1016/0166-3542(85)90037-3. [DOI] [PubMed] [Google Scholar]
- Horn M. E., Reed S. E., Taylor P. Role of viruses and bacteria in acute wheezy bronchitis in childhood: a study of sputum. Arch Dis Child. 1979 Aug;54(8):587–592. doi: 10.1136/adc.54.8.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longini I. M., Jr, Monto A. S., Koopman J. S. Statistical procedures for estimating the community probability of illness in family studies: rhinovirus and influenza. Int J Epidemiol. 1984 Mar;13(1):99–106. doi: 10.1093/ije/13.1.99. [DOI] [PubMed] [Google Scholar]
- Merigan T. C., Reed S. E., Hall T. S., Tyrrell D. A. Inhibition of respiratory virus infection by locally applied interferon. Lancet. 1973 Mar 17;1(7803):563–567. doi: 10.1016/s0140-6736(73)90714-9. [DOI] [PubMed] [Google Scholar]
- Meschievitz C. K., Schultz S. B., Dick E. C. A model for obtaining predictable natural transmission of rhinoviruses in human volunteers. J Infect Dis. 1984 Aug;150(2):195–201. doi: 10.1093/infdis/150.2.195. [DOI] [PubMed] [Google Scholar]
- Minor T. E., Dick E. C., Baker J. W., Ouellette J. J., Cohen M., Reed C. E. Rhinovirus and influenza type A infections as precipitants of asthma. Am Rev Respir Dis. 1976 Feb;113(2):149–153. doi: 10.1164/arrd.1976.113.2.149. [DOI] [PubMed] [Google Scholar]
- Monto A. S., Gunn R. A., Bandyk M. G., King C. L. Prevention of Russian influenza by amantadine. JAMA. 1979 Mar 9;241(10):1003–1007. [PubMed] [Google Scholar]
- Monto A. S., Shope T. C., Schwartz S. A., Albrecht J. K. Intranasal interferon-alpha 2b for seasonal prophylaxis of respiratory infection. J Infect Dis. 1986 Jul;154(1):128–133. doi: 10.1093/infdis/154.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samo T. C., Greenberg S. B., Couch R. B., Quarles J., Johnson P. E., Hook S., Harmon M. W. Efficacy and tolerance of intranasally applied recombinant leukocyte A interferon in normal volunteers. J Infect Dis. 1983 Sep;148(3):535–542. doi: 10.1093/infdis/148.3.535. [DOI] [PubMed] [Google Scholar]
- Samo T. C., Greenberg S. B., Palmer J. M., Couch R. B., Harmon M. W., Johnson P. E. Intranasally applied recombinant leukocyte A interferon in normal volunteers. II. Determination of minimal effective and tolerable dose. J Infect Dis. 1984 Aug;150(2):181–188. doi: 10.1093/infdis/150.2.181. [DOI] [PubMed] [Google Scholar]
- Scott G. M., Phillpotts R. J., Wallace J., Gauci C. L., Greiner J., Tyrrell D. A. Prevention of rhinovirus colds by human interferon alpha-2 from Escherichia coli. Lancet. 1982 Jul 24;2(8291):186–188. doi: 10.1016/s0140-6736(82)91031-5. [DOI] [PubMed] [Google Scholar]
- Smith C. B., Golden C. A., Kanner R. E., Renzetti A. D., Jr Association of viral and Mycoplasma pneumoniae infections with acute respiratory illness in patients with chronic obstructive pulmonary diseases. Am Rev Respir Dis. 1980 Feb;121(2):225–232. doi: 10.1164/arrd.1980.121.2.225. [DOI] [PubMed] [Google Scholar]
- TAYLOR-ROBINSON D., BYNOE M. L. INOCULATION OF VOLUNTEERS WITH H RHINOVIRUSES. Br Med J. 1964 Feb 29;1(5382):540–544. [PMC free article] [PubMed] [Google Scholar]
- Turner R. B., Felton A., Kosak K., Kelsey D. K., Meschievitz C. K. Prevention of experimental coronavirus colds with intranasal alpha-2b interferon. J Infect Dis. 1986 Sep;154(3):443–447. doi: 10.1093/infdis/154.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]


