Abstract
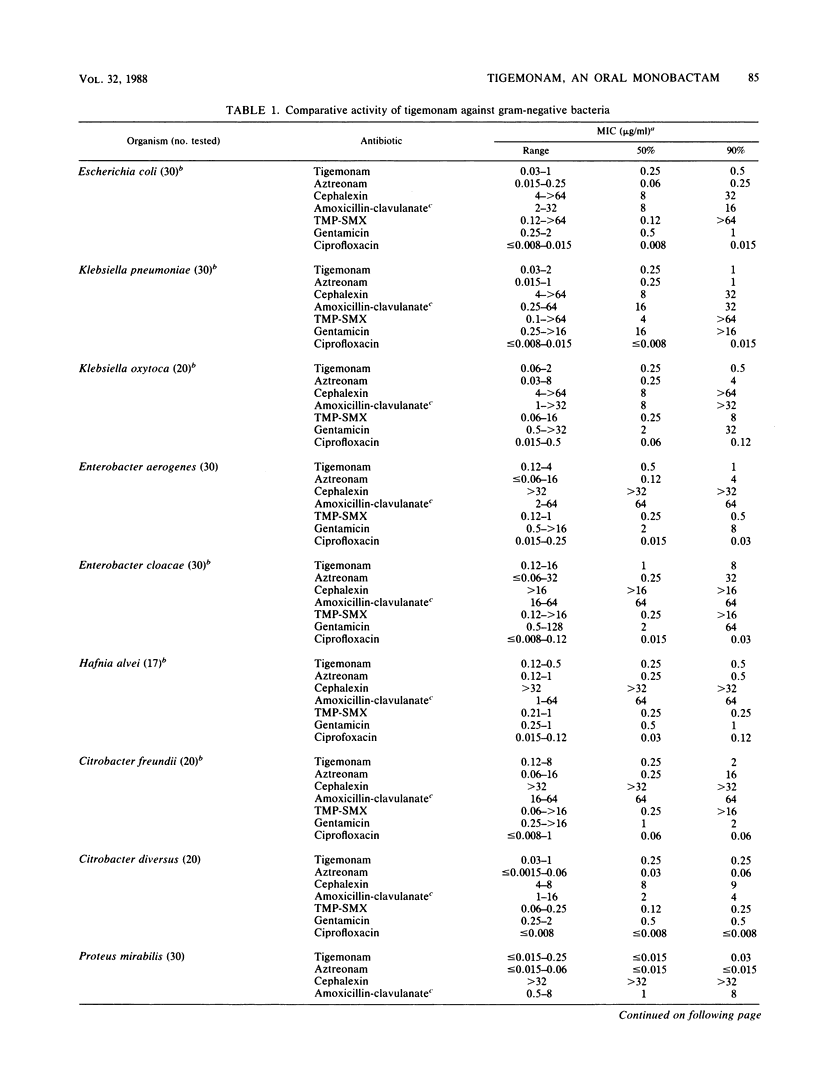
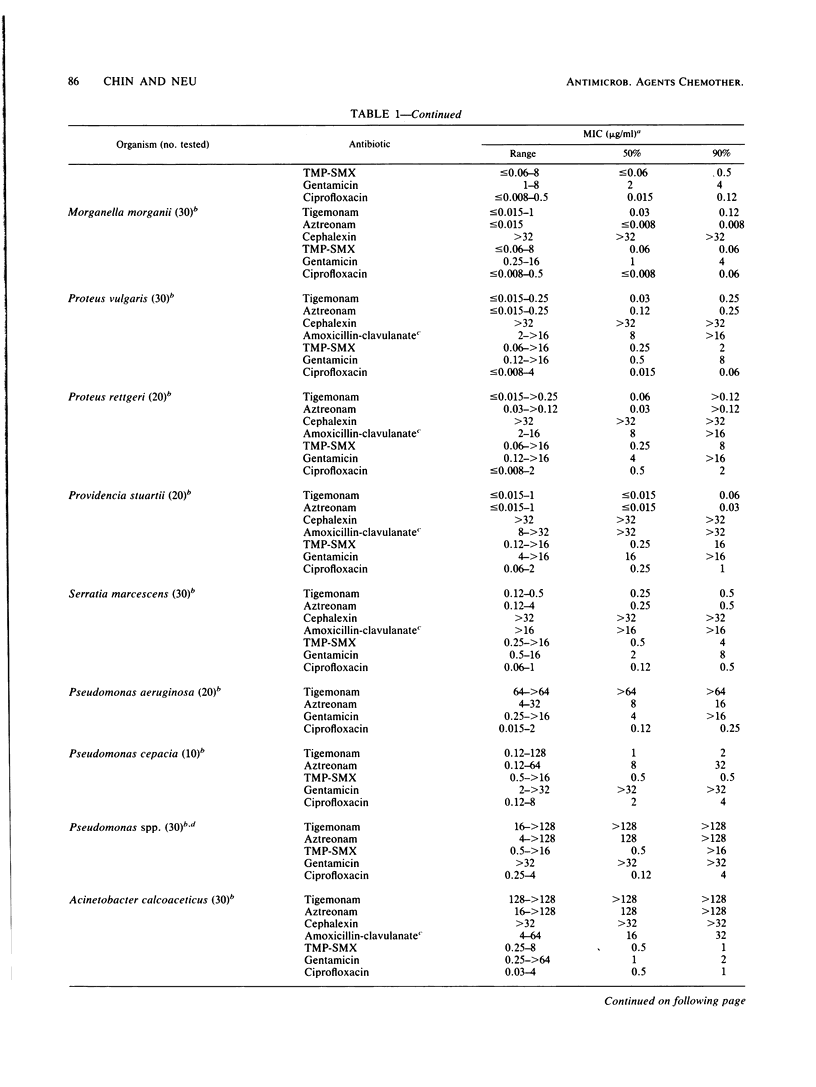
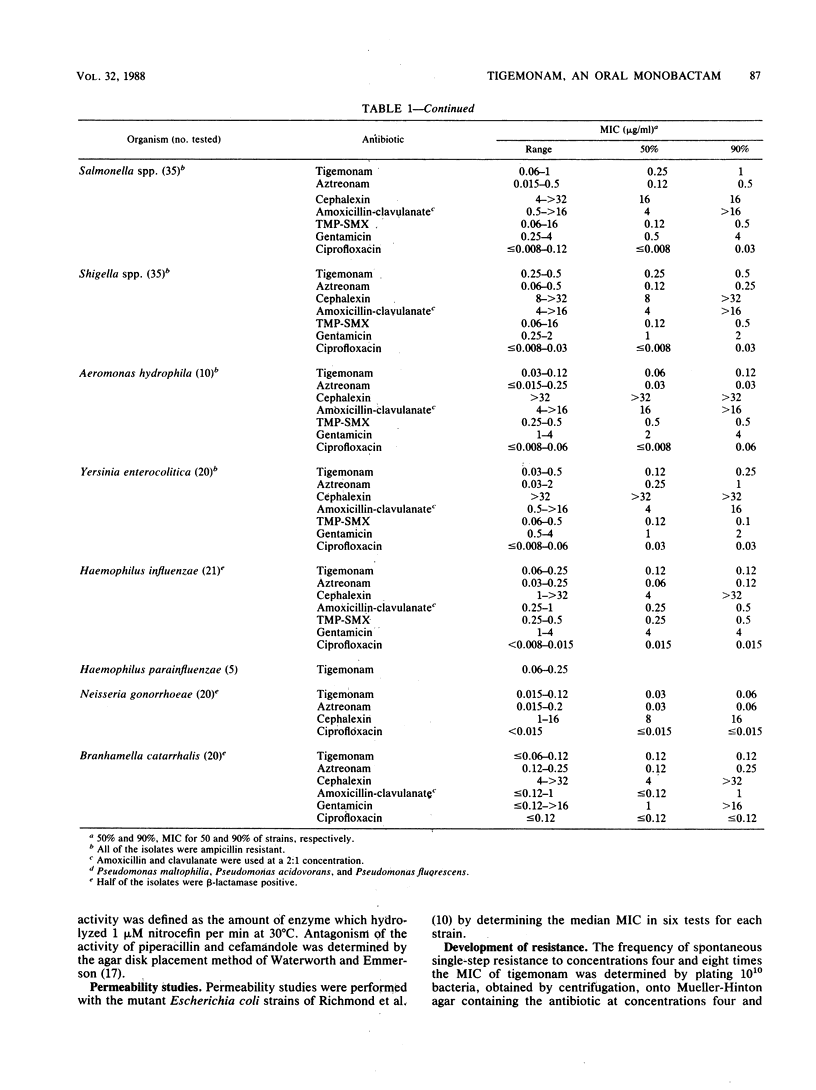
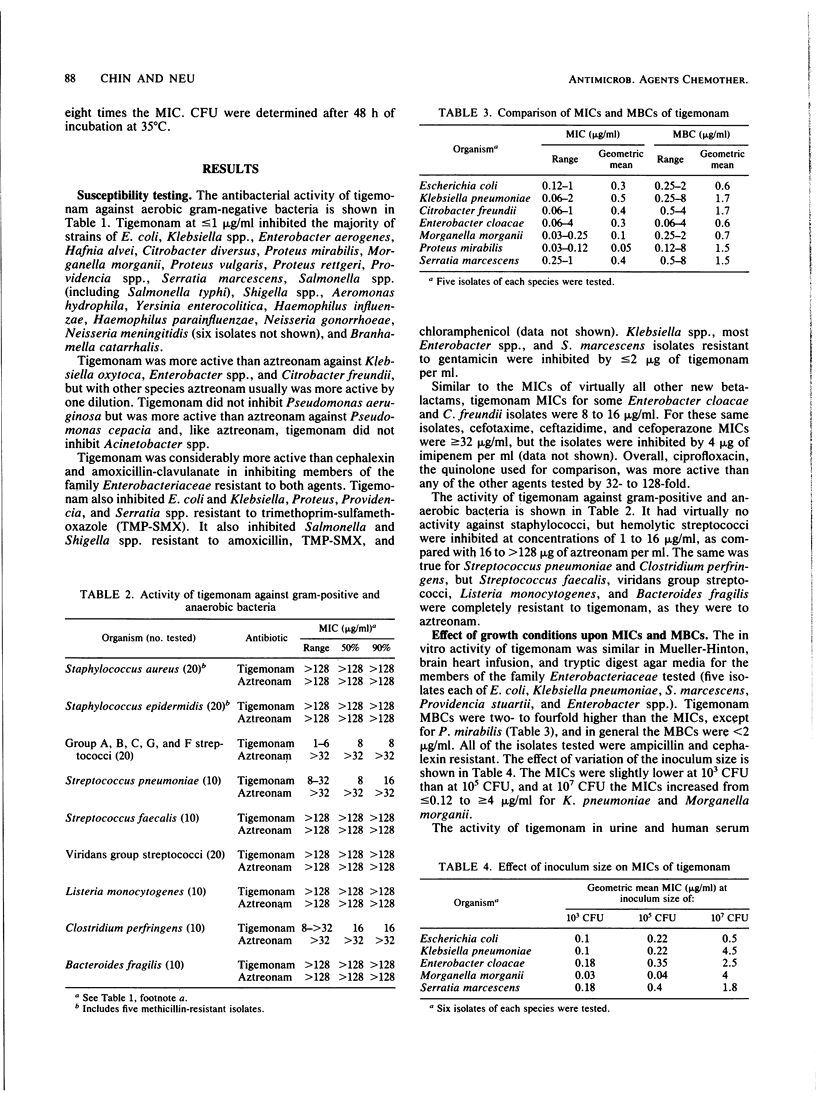
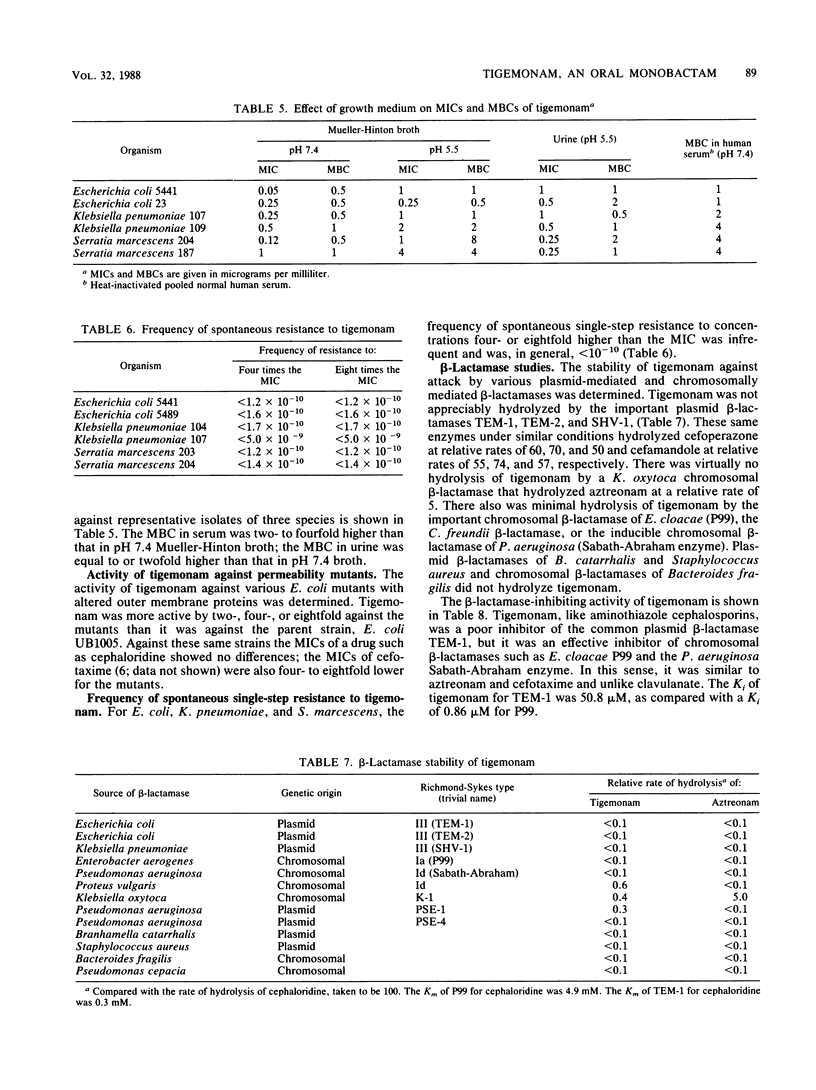
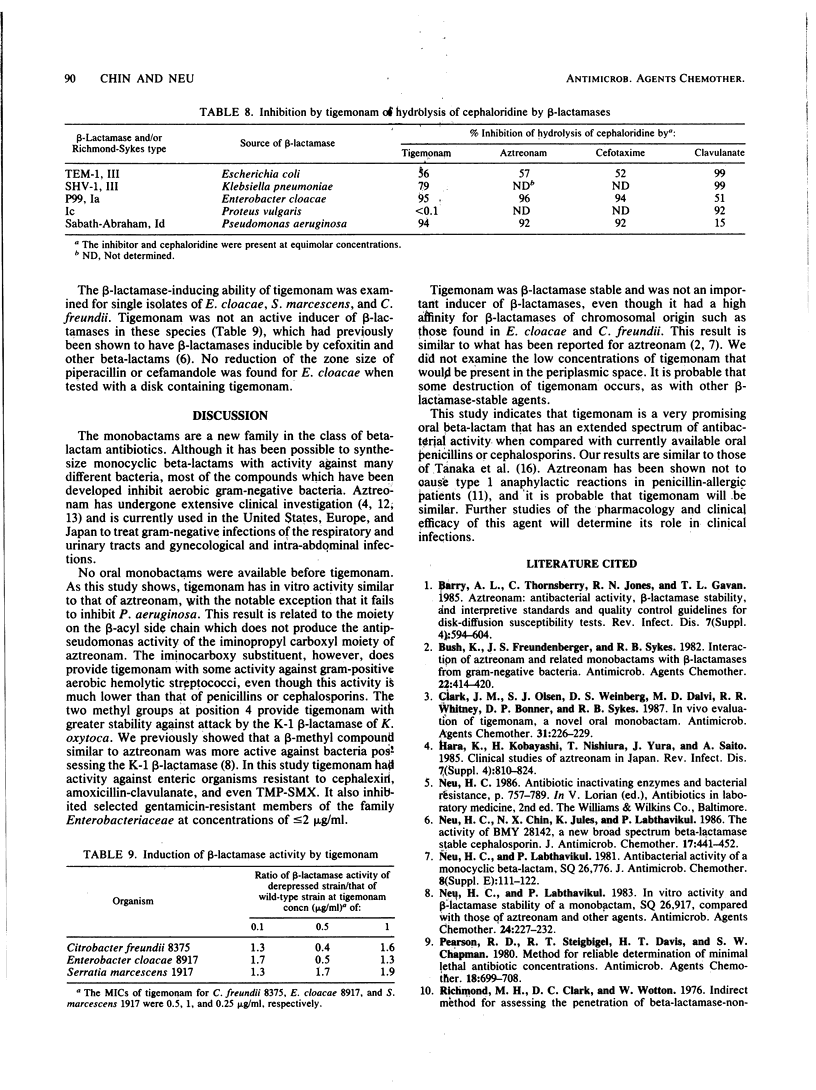
Tigemonam is an orally administered monobactam. At less than or equal to 1 microgram/ml it inhibited the majority of strains of Escherichia coli, Klebsiella spp., Enterobacter aerogenes, Citrobacter diversus, Proteus spp., Providencia spp., Aeromonas hydrophila, Salmonella spp., Shigella spp., Serratia marcescens, and Yersinia enterocolitica. At less than or equal to 0.25 microgram/ml it inhibited Haemophilus spp., Neisseria spp., and Branhamella catarrhalis. It did not inhibit Pseudomonas spp. or Acinetobacter spp. Tigemonam was more active than cephalexin and amoxicillin-clavulanate and inhibited many members of the family Enterobacteriaceae resistant to trimethoprim-sulfamethoxazole and gentamicin. Some Enterobacter cloacae and Citrobacter freundii strains resistant to aminothiazole iminomethoxy cephalosporins and aztreonam were resistant to tigemonam. The MIC for 90% of hemolytic streptococci of groups A, B, and C and for Streptococcus pneumoniae was 16 micrograms/ml, but the MIC for 90% of enterococci, Listeria spp., Bacteroides spp., and viridans group streptococci was greater than 64 micrograms/ml. Tigemonam was not hydrolyzed by the common plasmid beta-lactamases such as TEM-1 and SHV-1 or by the chromosomal beta-lactamases of Enterobacter, Morganella, Pseudomonas, and Bacteroides spp. Tigemonam inhibited beta-lactamases of E. cloacae and Pseudomonas aeruginosa but did not induce beta-lactamases. The growth medium had a minimal effect on the in vitro activity of tigemonam, and there was a close agreement between the MICs and MBCs.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bush K., Freudenberger J. S., Sykes R. B. Interaction of azthreonam and related monobactams with beta-lactamases from gram-negative bacteria. Antimicrob Agents Chemother. 1982 Sep;22(3):414–420. doi: 10.1128/aac.22.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J. M., Olsen S. J., Weinberg D. S., Dalvi M., Whitney R. R., Bonner D. P., Sykes R. B. In vivo evaluation of tigemonam, a novel oral monobactam. Antimicrob Agents Chemother. 1987 Feb;31(2):226–229. doi: 10.1128/aac.31.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene B. M., Gbakima A. A., Albiez E. J., Taylor H. R. Humoral and cellular immune responses to Onchocerca volvulus infection in humans. Rev Infect Dis. 1985 Nov-Dec;7(6):789–795. doi: 10.1093/clinids/7.6.789. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Chin N. X., Jules K., Labthavikul P. The activity of BMY 28142 a new broad spectrum beta-lactamase stable cephalosporin. J Antimicrob Chemother. 1986 Apr;17(4):441–452. doi: 10.1093/jac/17.4.441. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Antibacterial activity of a monocyclic beta-lactam SQ 26,776. J Antimicrob Chemother. 1981 Dec;8 (Suppl E):111–122. doi: 10.1093/jac/8.suppl_e.111. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. In vitro activity and beta-lactamase stability of a monobactam, SQ 26,917, compared with those of aztreonam and other agents. Antimicrob Agents Chemother. 1983 Aug;24(2):227–232. doi: 10.1128/aac.24.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Clark D. C., Wotton S. Indirect method for assessing the penetration of beta-lactamase-nonsusceptible penicillins and cephalosporins in Escherichia coli strains. Antimicrob Agents Chemother. 1976 Aug;10(2):215–218. doi: 10.1128/aac.10.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Swabb E. A., Adkinson N. F., Jr Investigation into the immunologic cross-reactivity of aztreonam with other beta-lactam antibiotics. Am J Med. 1985 Feb 8;78(2A):19–26. doi: 10.1016/0002-9343(85)90198-6. [DOI] [PubMed] [Google Scholar]
- Scully B. E., Neu H. C. Use of aztreonam in the treatment of serious infections due to multiresistant gram-negative organisms, including Pseudomonas aeruginosa. Am J Med. 1985 Feb;78(2):251–261. doi: 10.1016/0002-9343(85)90435-8. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S. K., Summerill R. A., Minassian B. F., Bush K., Visnic D. A., Bonner D. P., Sykes R. B. In vitro evaluation of tigemonam, a novel oral monobactam. Antimicrob Agents Chemother. 1987 Feb;31(2):219–225. doi: 10.1128/aac.31.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]


