Abstract
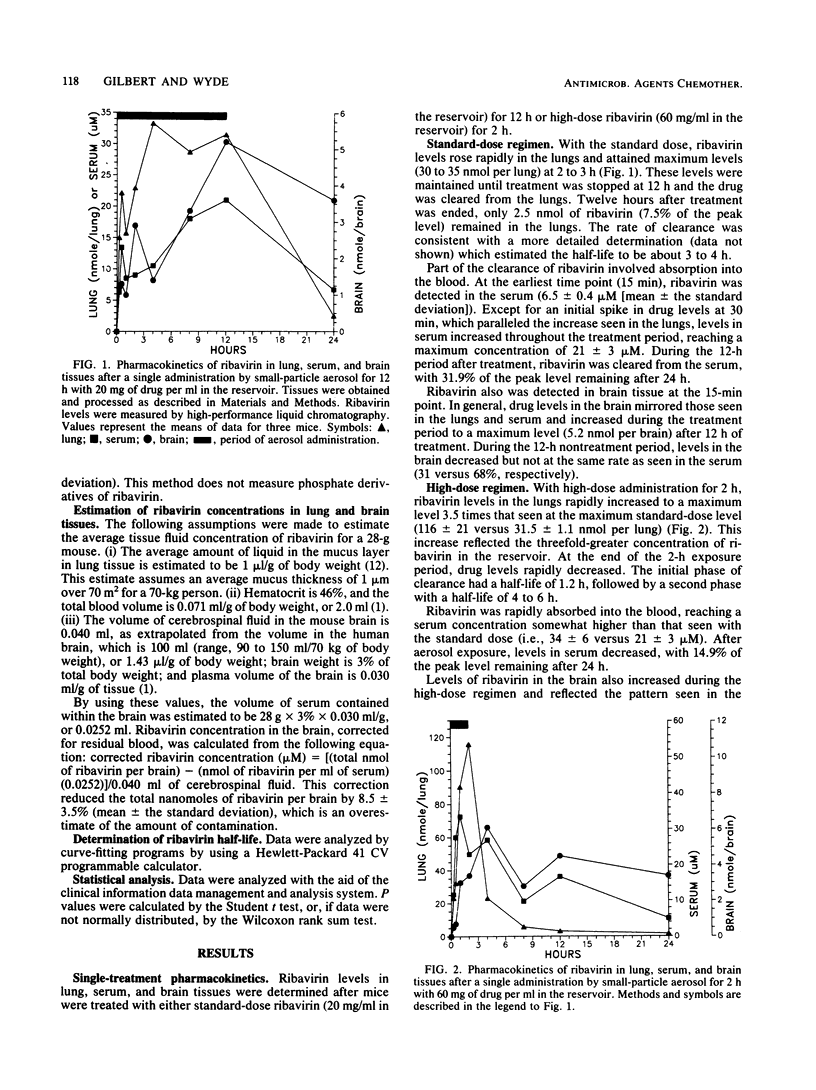
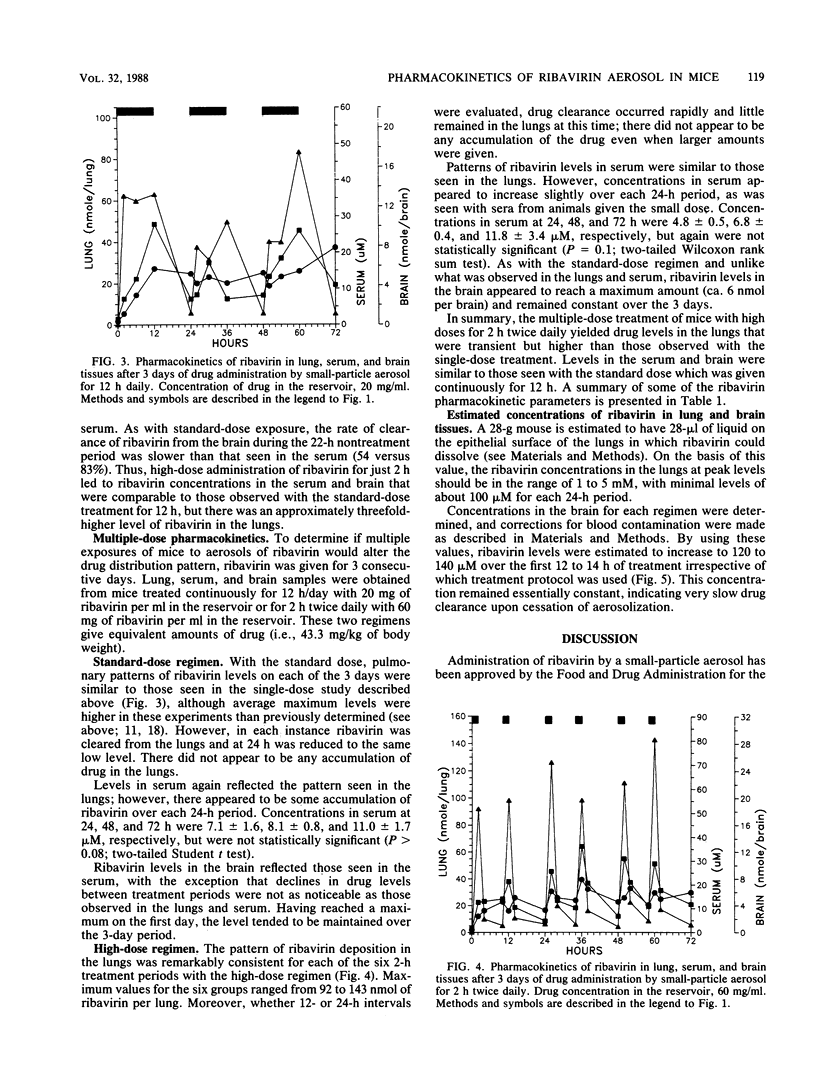
The pharmacokinetics of ribavirin administered in single or multiple treatments to mice by small-particle aerosol were monitored in lung, serum, and brain tissues. ribavirin aerosol was administered with a standard drug concentration (20 mg/ml) in the reservoir for 12 h or a high dose (60 mg/ml) for 2 or 4 h. After single or 3-day treatments, ribavirin rapidly accumulated in the lungs at concentrations sufficient to inhibit influenza virus or respiratory syncytial virus (1 to 5 mM). While peak levels of ribavirin in the lungs after the high-dose administration were about three times those found with the standard dose, ribavirin was rapidly cleared from the lungs. There was no accumulation of drug in the lungs after multiple treatments. Ribavirin cleared from the lungs was detected in the blood within 15 min. Concentrations in the serum were similar (20 to 30 microM) for standard- and high-dose treatments with either single or multiple treatments. Ribavirin clearance from the serum after treatment was similar for each regimen. Ribavirin also rapidly accumulated in the brain to a similar level (ca. 6 nmol per brain) after standard- or high-dose treatment for 3 days. In contrast to ribavirin in the serum, ribavirin in the brain appeared to be slowly cleared, allowing levels to remain relatively constant during and after treatment. With the interest in viral encephalopathies, further evaluation of the possible advantages of this method of drug administration is warranted.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin R. K., Trefts P. E., Hintz M., Connor J. D., Kagnoff M. F. Sensitive radioimmunoassay for the broad-spectrum antiviral agent ribavirin. Antimicrob Agents Chemother. 1983 Nov;24(5):696–701. doi: 10.1128/aac.24.5.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C., Bubley G., Lucey D., Hussey S., Connor J. Ribavirin enters cerebrospinal fluid. Lancet. 1986 Jul 5;2(8497):45–46. doi: 10.1016/s0140-6736(86)92590-0. [DOI] [PubMed] [Google Scholar]
- Ferrara E. A., Oishi J. S., Wannemacher R. W., Jr, Stephen E. L. Plasma disappearance, urine excretion, and tissue distribution of ribavirin in rats and rhesus monkeys. Antimicrob Agents Chemother. 1981 Jun;19(6):1042–1049. doi: 10.1128/aac.19.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand E. W., McCurdy D., Rao C. P., Middleton P. J. Ribavirin treatment of viral pneumonitis in severe combined immunodeficiency disease. Lancet. 1983 Sep 24;2(8352):732–733. doi: 10.1016/s0140-6736(83)92265-1. [DOI] [PubMed] [Google Scholar]
- Gilbert B. E., Knight V. Biochemistry and clinical applications of ribavirin. Antimicrob Agents Chemother. 1986 Aug;30(2):201–205. doi: 10.1128/aac.30.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert B. E., Wilson S. Z., Knight V., Couch R. B., Quarles J. M., Dure L., Hayes N., Willis G. Ribavirin small-particle aerosol treatment of infections caused by influenza virus strains A/Victoria/7/83 (H1N1) and B/Texas/1/84. Antimicrob Agents Chemother. 1985 Mar;27(3):309–313. doi: 10.1128/aac.27.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C. B., McBride J. T., Walsh E. E., Bell D. M., Gala C. L., Hildreth S., Ten Eyck L. G., Hall W. J. Aerosolized ribavirin treatment of infants with respiratory syncytial viral infection. A randomized double-blind study. N Engl J Med. 1983 Jun 16;308(24):1443–1447. doi: 10.1056/NEJM198306163082403. [DOI] [PubMed] [Google Scholar]
- Lippmann M., Yeates D. B., Albert R. E. Deposition, retention, and clearance of inhaled particles. Br J Ind Med. 1980 Nov;37(4):337–362. doi: 10.1136/oem.37.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick J. B., Getchell J. P., Mitchell S. W., Hicks D. R. Ribavirin suppresses replication of lymphadenopathy-associated virus in cultures of human adult T lymphocytes. Lancet. 1984 Dec 15;2(8416):1367–1369. doi: 10.1016/s0140-6736(84)92060-9. [DOI] [PubMed] [Google Scholar]
- Smith R. H., Gilbert B. E. Quantification of ribavirin in biological fluids and tissues by high-performance liquid chromatography. J Chromatogr. 1987 Feb 20;414(1):202–210. doi: 10.1016/0378-4347(87)80042-7. [DOI] [PubMed] [Google Scholar]
- Wilson S. Z., Gilbert B. E., Quarles J. M., Knight V., McClung H. W., Moore R. V., Couch R. B. Treatment of influenza A (H1N1) virus infection with ribavirin aerosol. Antimicrob Agents Chemother. 1984 Aug;26(2):200–203. doi: 10.1128/aac.26.2.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. Z., Knight V., Wyde P. R., Drake S., Couch R. B. Amantadine and ribavirin aerosol treatment of influenza A and B infection in mice. Antimicrob Agents Chemother. 1980 Apr;17(4):642–648. doi: 10.1128/aac.17.4.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Wilson S. Z., Gilbert B. E., Smith R. H. Protection of mice from lethal influenza virus infection with high dose-short duration ribavirin aerosol. Antimicrob Agents Chemother. 1986 Dec;30(6):942–944. doi: 10.1128/aac.30.6.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyde P. R., Wilson S. Z., Petrella R., Gilbert B. E. Efficacy of high dose-short duration ribavirin aerosol in the treatment of respiratory syncytial virus infected cotton rats and influenza B virus infected mice. Antiviral Res. 1987 May;7(4):211–220. doi: 10.1016/0166-3542(87)90029-5. [DOI] [PubMed] [Google Scholar]


