Abstract
AIMS—To assess the cellular proliferation using the monoclonal antibody Ki-67, in paraffin embedded uveal melanomas irradiated by proton beam, as well as in non-irradiated uveal melanomas. METHODS—30 enucleated eyes were included for histopathological study and Ki-67 immunostaining. Patients were enucleated between 1991 and 1996 for uveal melanoma, 14 after proton beam irradiation and 16 without treatment (control group). The mean follow up period was 2.5 years after diagnosis and 1 year after enucleation. RESULTS—A significant relation was found between Ki-67 score and mitotic index (r = 0.56, p = 0.001), histological largest tumour diameter (r = 0.38, p = 0.03), fibrosis (r = −0.35, p = 0.05), absence of tumoral pigmentation (p = 0.05), and presence of vascular thrombosis (p = 0.03). The Ki-67 score was significantly higher in the non-irradiated group (p = 0.01) and in the group of patients whose cause of enucleation was tumoral evolution (p = 0.005) compared with the group of patients enucleated after neovascular glaucoma. The Ki-67 score was very high in a case of orbital recurrence of uveal melanoma and metastatic death. 70% of metastasised tumours showed a Ki-67 score higher than the median value. CONCLUSION—Ki-67 labelling is a reliable method of estimating the proliferative activity in uveal melanomas after proton beam irradiation. The Ki-67 score is significantly correlated with prognostic variables (mitotic index and histological largest tumour diameter), and with radiation effects after proton beam irradiation.
Full Text
The Full Text of this article is available as a PDF (162.9 KB).
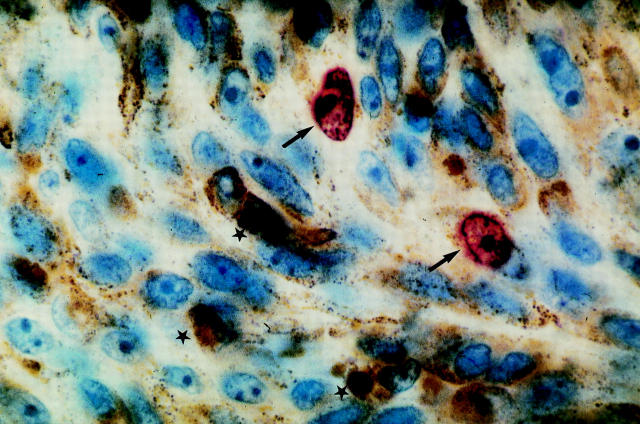
Figure 1 .
Ki-67 immunostaining in an irradiated choroidal spindle cell melanoma. Note the speckled red nuclear and nucleolar staining (arrows) readily distinguished from the brown endogenous melanin pigment (stars) (original magnification ×1000).
Figure 2 .
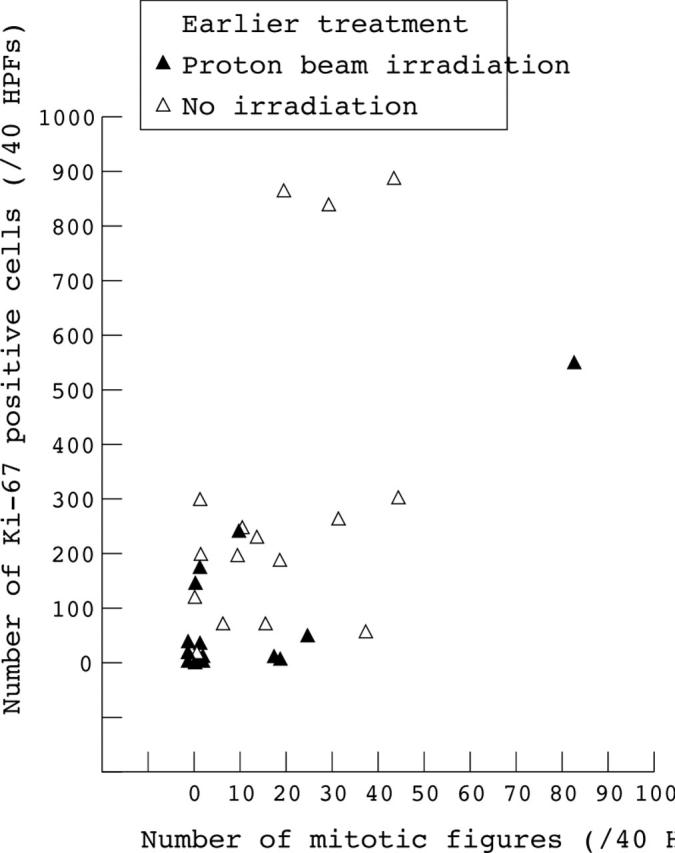
Mitotic figures plotted against Ki-67 positive cells for 30 eyes. Filled symbols represent data for tumours treated by proton beam irradiation and open symbols, data for non-irradiated tumours. The mitotic figures and Ki-67 immunoreactive cells were counted in 40 high power fields (HPFs).
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardenstein D. S., Char D. H., Kaleta-Michaels S., Kroll S. M. Ki-67 and bromodeoxyuridine labeling of human choroidal melanoma cells. Curr Eye Res. 1991 May;10(5):479–484. doi: 10.3109/02713689109001755. [DOI] [PubMed] [Google Scholar]
- Brown D. C., Gatter K. C. Monoclonal antibody Ki-67: its use in histopathology. Histopathology. 1990 Dec;17(6):489–503. doi: 10.1111/j.1365-2559.1990.tb00788.x. [DOI] [PubMed] [Google Scholar]
- Char D. H., Huhta K., Waldman F. DNA cell cycle studies in uveal melanoma. Am J Ophthalmol. 1989 Jan 15;107(1):65–72. doi: 10.1016/0002-9394(89)90817-9. [DOI] [PubMed] [Google Scholar]
- Chen T. C., Char D. H., Waldman F., Juster R. P. Flow cytometry measurement of nuclear RNA content in uveal melanoma. Ophthalmic Res. 1990;22(3):187–193. doi: 10.1159/000267022. [DOI] [PubMed] [Google Scholar]
- Coleman K., Baak J. P., van Diest P. J., Curran B., Mullaney J., Fenton M., Leader M. DNA ploidy status in 84 ocular melanomas: a study of DNA quantitation in ocular melanomas by flow cytometry and automatic and interactive static image analysis. Hum Pathol. 1995 Jan;26(1):99–105. doi: 10.1016/0046-8177(95)90121-3. [DOI] [PubMed] [Google Scholar]
- Coupland S. E., Bechrakis N., Schüler A., Anagnostopoulos I., Hummel M., Bornfeld N., Stein H. Expression patterns of cyclin D1 and related proteins regulating G1-S phase transition in uveal melanoma and retinoblastoma. Br J Ophthalmol. 1998 Aug;82(8):961–970. doi: 10.1136/bjo.82.8.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener-West M., Hawkins B. S., Markowitz J. A., Schachat A. P. A review of mortality from choroidal melanoma. II. A meta-analysis of 5-year mortality rates following enucleation, 1966 through 1988. Arch Ophthalmol. 1992 Feb;110(2):245–250. doi: 10.1001/archopht.1992.01080140101036. [DOI] [PubMed] [Google Scholar]
- Egan K. M., Seddon J. M., Glynn R. J., Gragoudas E. S., Albert D. M. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol. 1988 Jan-Feb;32(4):239–251. doi: 10.1016/0039-6257(88)90173-7. [DOI] [PubMed] [Google Scholar]
- Gamel J. W., McLean I. W. Computerized histopathologic assessment of malignant potential. II. A practical method for predicting survival following enucleation for uveal melanoma. Cancer. 1983 Sep 15;52(6):1032–1038. doi: 10.1002/1097-0142(19830915)52:6<1032::aid-cncr2820520618>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Gerdes J., Lemke H., Baisch H., Wacker H. H., Schwab U., Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol. 1984 Oct;133(4):1710–1715. [PubMed] [Google Scholar]
- Gerdes J., Schwab U., Lemke H., Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983 Jan 15;31(1):13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Ghazvini S., Kroll S., Char D. H., Frigillana H. Comparative analysis of proliferating cell nuclear antigen, bromodeoxyuridine, and mitotic index in uveal melanoma. Invest Ophthalmol Vis Sci. 1995 Dec;36(13):2762–2767. [PubMed] [Google Scholar]
- Hall P. A., Levison D. A. Review: assessment of cell proliferation in histological material. J Clin Pathol. 1990 Mar;43(3):184–192. doi: 10.1136/jcp.43.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Levison D. A., Woods A. L., Yu C. C., Kellock D. B., Watkins J. A., Barnes D. M., Gillett C. E., Camplejohn R., Dover R. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990 Dec;162(4):285–294. doi: 10.1002/path.1711620403. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Karlsson M., Boeryd B., Carstensen J., Frånlund B., Gustafsson B., Kågedal B., Sun X. F., Wingren S. Correlations of Ki-67 and PCNA to DNA ploidy, S-phase fraction and survival in uveal melanoma. Eur J Cancer. 1996 Feb;32A(2):357–362. doi: 10.1016/0959-8049(95)00562-5. [DOI] [PubMed] [Google Scholar]
- Karlsson M., Boeryd B., Carstensen J., Kågedal B., Wingren S. DNA ploidy and S-phase fraction as prognostic factors in patients with uveal melanomas. Br J Cancer. 1995 Jan;71(1):177–181. doi: 10.1038/bjc.1995.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M. K. Balloon cell malignant melanoma of the choroid: ultrastructural studies. Br J Ophthalmol. 1983 Sep;67(9):579–584. doi: 10.1136/bjo.67.9.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Foster W. D., Zimmerman L. E., Gamel J. W. Modifications of Callender's classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983 Oct;96(4):502–509. doi: 10.1016/s0002-9394(14)77914-0. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Gamel J. W. Prediction of metastasis of uveal melanoma: comparison of morphometric determination of nucleolar size and spectrophotometric determination of DNA. Invest Ophthalmol Vis Sci. 1988 Apr;29(4):507–511. [PubMed] [Google Scholar]
- Meecham W. J., Char D. H. DNA content abnormalities and prognosis in uveal melanoma. Arch Ophthalmol. 1986 Nov;104(11):1626–1629. doi: 10.1001/archopht.1986.01050230064033. [DOI] [PubMed] [Google Scholar]
- Mooy C. M., Luyten G. P., de Jong P. T., Luider T. M., Stijnen T., van de Ham F., van Vroonhoven C. C., Bosman F. T. Immunohistochemical and prognostic analysis of apoptosis and proliferation in uveal melanoma. Am J Pathol. 1995 Oct;147(4):1097–1104. [PMC free article] [PubMed] [Google Scholar]
- Mooy C. M., de Jong P. T., Van der Kwast T. H., Mulder P. G., Jager M. J., Ruiter D. J. Ki-67 immunostaining in uveal melanoma. The effect of pre-enucleation radiotherapy. Ophthalmology. 1990 Oct;97(10):1275–1280. doi: 10.1016/s0161-6420(90)32420-x. [DOI] [PubMed] [Google Scholar]
- Mooy C., Vissers K., Luyten G., Mulder A., Stijnen T., de Jong P., Bosman F. DNA flow cytometry in uveal melanoma: the effect of pre-enucleation irradiation. Br J Ophthalmol. 1995 Feb;79(2):174–177. doi: 10.1136/bjo.79.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag C. B., Volk B., Shibata T., Burger P., Kleihues P. The monoclonal antibody Ki-67 as a marker for proliferating cells in stereotactic biopsies of brain tumours. Acta Neurochir (Wien) 1987;89(3-4):117–121. doi: 10.1007/BF01560376. [DOI] [PubMed] [Google Scholar]
- Pe'er J., Gnessin H., Shargal Y., Livni N. PC-10 immunostaining of proliferating cell nuclear antigen in posterior uveal melanoma. Enucleation versus enucleation postirradiation groups. Ophthalmology. 1994 Jan;101(1):56–62. doi: 10.1016/s0161-6420(94)38024-9. [DOI] [PubMed] [Google Scholar]
- Saornil M. A., Egan K. M., Gragoudas E. S., Seddon J. M., Walsh S. M., Albert D. M. Histopathology of proton beam-irradiated vs enucleated uveal melanomas. Arch Ophthalmol. 1992 Aug;110(8):1112–1118. doi: 10.1001/archopht.1992.01080200092031. [DOI] [PubMed] [Google Scholar]
- Saornil M. A., Fisher M. R., Campbell R. J., Robertson D. M., Earle J. D., Eagle R. C., Jr, Shields J. A., Shields C. L., Chandra S. R., Albert D. M. Histopathologic study of eyes after iodine I 125 episcleral plaque irradiation for uveal melanoma. Arch Ophthalmol. 1997 Nov;115(11):1395–1400. doi: 10.1001/archopht.1997.01100160565006. [DOI] [PubMed] [Google Scholar]
- Schilling H., Sehu K. W., Lee W. R. A histologic study (including DNA quantification and Ki-67 labeling index) in uveal melanomas after brachytherapy with ruthenium plaques. Invest Ophthalmol Vis Sci. 1997 Sep;38(10):2081–2092. [PubMed] [Google Scholar]
- Seddon J. M., Polivogianis L., Hsieh C. C., Albert D. M., Gamel J. W., Gragoudas E. S. Death from uveal melanoma. Number of epithelioid cells and inverse SD of nucleolar area as prognostic factors. Arch Ophthalmol. 1987 Jun;105(6):801–806. doi: 10.1001/archopht.1987.01060060087039. [DOI] [PubMed] [Google Scholar]
- Seregard S., Lundell G., Lax I., af Trampe E., Kock E. Tumour cell proliferation after failed ruthenium plaque radiotherapy for posterior uveal melanoma. Acta Ophthalmol Scand. 1997 Apr;75(2):148–154. doi: 10.1111/j.1600-0420.1997.tb00112.x. [DOI] [PubMed] [Google Scholar]
- Seregard S., Oskarsson M., Spångberg B. PC-10 as a predictor of prognosis after antigen retrieval in posterior uveal melanoma. Invest Ophthalmol Vis Sci. 1996 Jun;37(7):1451–1458. [PubMed] [Google Scholar]
- Seregard S., Spångberg B., Juul C., Oskarsson M. Prognostic accuracy of the mean of the largest nucleoli, vascular patterns, and PC-10 in posterior uveal melanoma. Ophthalmology. 1998 Mar;105(3):485–491. doi: 10.1016/S0161-6420(98)93032-9. [DOI] [PubMed] [Google Scholar]