Abstract
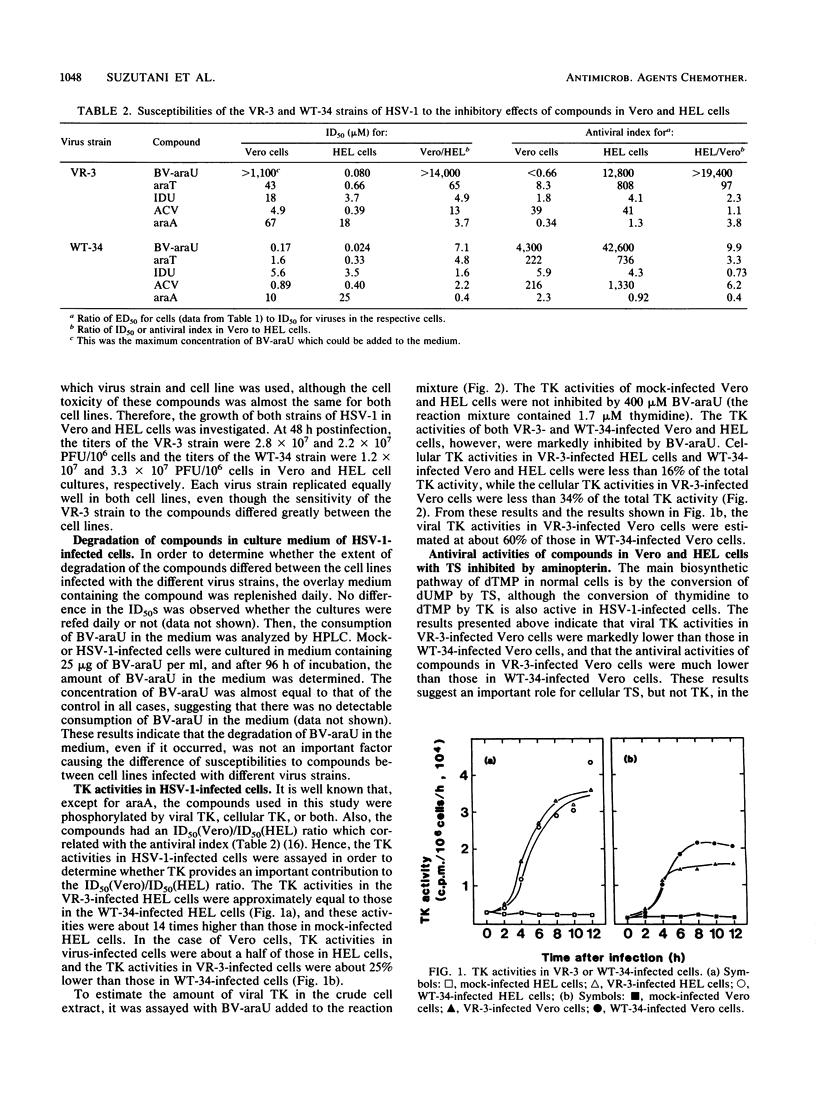
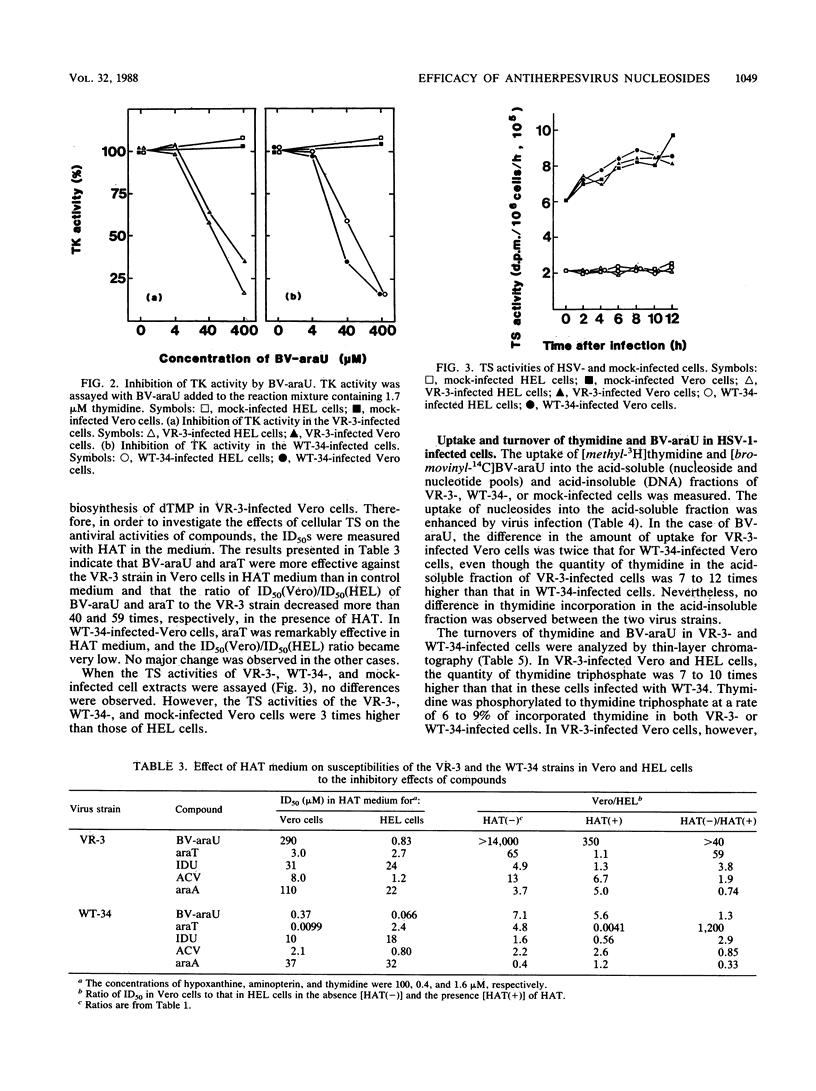
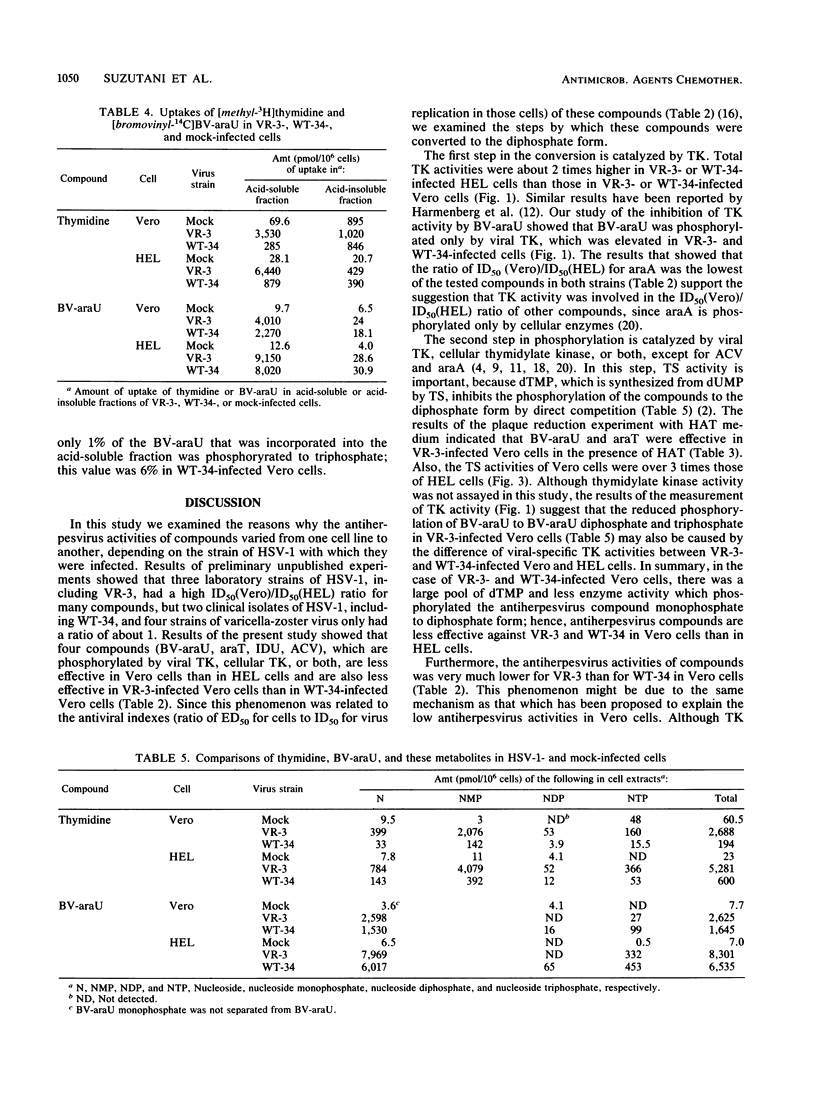
Antiviral activities of five nucleoside analogs against the VR-3 and WT-34 strains of herpes simplex virus type 1 (HSV-1) were investigated in Vero and human embryo lung fibroblast (HEL) cells. In HEL cells, the compounds showed antiviral activities against both strains of HSV-1, but in Vero cells, the antiviral activities of the compounds were reduced in proportion to their antiviral indexes (the 50% inhibitory dose [ID50] for cell growth divided by the 50% plaque reduction dose for virus). The ratio of the ID50 in Vero cells to the ID50 in HEL cells was larger in VR-3-infected cells than in WT-34-infected cells. The following results were obtained. (i) Thymidine kinase (TK; EC 2.7.1.21) activity in the VR-3- or WT-34-infected Vero cells was about half that in VR-3- or WT-34-infected HEL cells. Induction of viral TK was especially low in the VR-3-infected Vero cells. (ii) The ID50 of the plaque reduction assay in hypoxanthine, aminopterin, and thymidine medium revealed that the activity of cellular thymidylate synthetase (EC 2.1.1.45) was important in viral replication in VR-3-infected Vero cells. (iii) The VR-3-infected cells required larger thymidine and thymidine phosphate pools for viral replication than the WT-34-infected cells did, although uptake of 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl) uracil into infected cells was equal for both strains. (iv) In the VR-3-infected Vero cells, the quantity of 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil triphosphate was smaller than that in VR-3-infected HEL cells and WT-34-infected Vero and HEL cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aron G. M., Purifoy D. J., Schaffer P. A. DNA synthesis and DNA polymerase activity of herpes simplex virus type 1 temperature-sensitive mutants. J Virol. 1975 Sep;16(3):498–507. doi: 10.1128/jvi.16.3.498-507.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayisi N. K., Wall R. A., Wanklin R. J., Machida H., De Clercq E., Sacks S. L. Comparative metabolism of E-5-(2-bromovinyl)-2'-deoxyuridine and 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil in herpes simplex virus-infected cells. Mol Pharmacol. 1987 Apr;31(4):422–429. [PubMed] [Google Scholar]
- Ayusawa D., Iwata K., Seno T., Koyama H. Conditional thymidine auxotrophic mutants of mouse FM3A cells due to thermosensitive thymidylate synthase and their prototrophic revertants. J Biol Chem. 1981 Dec 10;256(23):12005–12012. [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Association of thymidylate kinase activity with pyrimidine deoxyribonucleoside kinase induced by herpes simplex virus. J Biol Chem. 1978 Mar 10;253(5):1325–1327. [PubMed] [Google Scholar]
- Coen D. M., Aschman D. P., Gelep P. T., Retondo M. J., Weller S. K., Schaffer P. A. Fine mapping and molecular cloning of mutations in the herpes simplex virus DNA polymerase locus. J Virol. 1984 Jan;49(1):236–247. doi: 10.1128/jvi.49.1.236-247.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Fleming H. E., Jr, Leslie L. K., Retondo M. J. Sensitivity of arabinosyladenine-resistant mutants of herpes simplex virus to other antiviral drugs and mapping of drug hypersensitivity mutations to the DNA polymerase locus. J Virol. 1985 Feb;53(2):477–488. doi: 10.1128/jvi.53.2.477-488.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clercq E. Comparative efficacy of antiherpes drugs in different cell lines. Antimicrob Agents Chemother. 1982 Apr;21(4):661–663. doi: 10.1128/aac.21.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowdle W. R., Nahmias A. J., Harwell R. W., Pauls F. P. Association of antigenic type of Herpesvirus hominis with site of viral recovery. J Immunol. 1967 Nov;99(5):974–980. [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A. Differential phosphorylation of (E)-5-(2-bromovinyl)-2'-deoxyuridine monophosphate by thymidylate kinases from herpes simplex viruses types 1 and 2 and varicella zoster virus. Mol Pharmacol. 1982 Mar;21(2):432–437. [PubMed] [Google Scholar]
- Harmenberg J., Källander C. F., Gronowitz J. S. Effect of acyclovir and presence of cellular and viral thymidine kinase activity in herpes simplex virus infected cells. Brief report. Arch Virol. 1982;74(2-3):219–225. doi: 10.1007/BF01314715. [DOI] [PubMed] [Google Scholar]
- Harmenberg J., Wahren B., Oberg B. Influence of cells and virus multiplicity on the inhibition of herpesviruses with acycloguanosine. Intervirology. 1980;14(5-6):239–244. doi: 10.1159/000149192. [DOI] [PubMed] [Google Scholar]
- Jamieson A. T., Gentry G. A., Subak-Sharpe J. H. Induction of both thymidine and deoxycytidine kinase activity by herpes viruses. J Gen Virol. 1974 Sep;24(3):465–480. doi: 10.1099/0022-1317-24-3-465. [DOI] [PubMed] [Google Scholar]
- Larsson A., Brännström G., Oberg B. Kinetic analysis in cell culture of the reversal of antiherpes activity of nucleoside analogs by thymidine. Antimicrob Agents Chemother. 1983 Nov;24(5):819–822. doi: 10.1128/aac.24.5.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida H. Comparison of susceptibilities of varicella-zoster virus and herpes simplex viruses to nucleoside analogs. Antimicrob Agents Chemother. 1986 Mar;29(3):524–526. doi: 10.1128/aac.29.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren C., Chen M. S., Ghazzouli I., Saral R., Burns W. H. Drug resistance patterns of herpes simplex virus isolates from patients treated with acyclovir. Antimicrob Agents Chemother. 1985 Dec;28(6):740–744. doi: 10.1128/aac.28.6.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980 Aug 10;255(15):7204–7207. [PubMed] [Google Scholar]
- Plunkett W., Cohen S. S. Two approaches that increase the activity of analogs of adenine nucleosides in animal cells. Cancer Res. 1975 Jun;35(6):1547–1554. [PubMed] [Google Scholar]
- Purifoy D. J., Benyesh-Melnick M. DNA polymerase induction by DNA-negative temperature-sensitive mutants of herpes simplex virus type 2. Virology. 1975 Dec;68(2):374–386. doi: 10.1016/0042-6822(75)90280-9. [DOI] [PubMed] [Google Scholar]
- Reefschläger J., Herrmann G., Bärwolff D., Schwarz B., Cech D., Langen P. Antiherpes activity of (E)-5-(2-bromovinyl)- and 5-vinyl-1-beta-D-arabinofuranosyluracil and some other 5-substituted uracil arabinosyl nucleosides in two different cell lines. Antiviral Res. 1983 Sep;3(3):175–187. doi: 10.1016/0166-3542(83)90024-4. [DOI] [PubMed] [Google Scholar]
- Roberts D. An isotopic assay for thymidylate synthetase. Biochemistry. 1966 Nov;5(11):3546–3548. doi: 10.1021/bi00875a022. [DOI] [PubMed] [Google Scholar]
- Sakuma T. Strains of varicella-zoster virus resistant to 1-beta-D-arabinofuranosyl-E-5-(2-bromovinyl)uracil. Antimicrob Agents Chemother. 1984 Jun;25(6):742–746. doi: 10.1128/aac.25.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Machida H., Saneyoshi M. Antiviral activity of various 1-beta-D-arabinofuranosyl-E-5-halogenovinyluracils and E-5-bromovinyl-2'-deoxyuridine against salmon herpes virus, Oncorhynchus masou virus (OMV). Antiviral Res. 1987 Feb;7(2):79–86. doi: 10.1016/0166-3542(87)90023-4. [DOI] [PubMed] [Google Scholar]


