Abstract
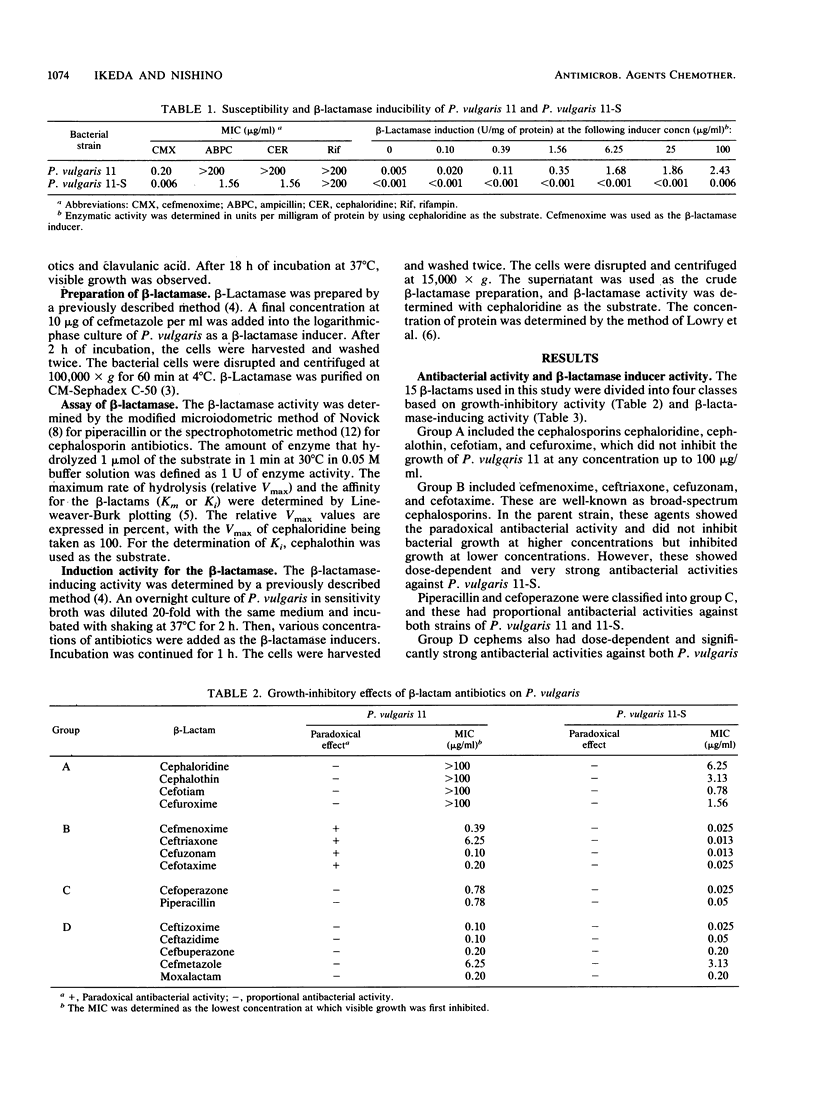
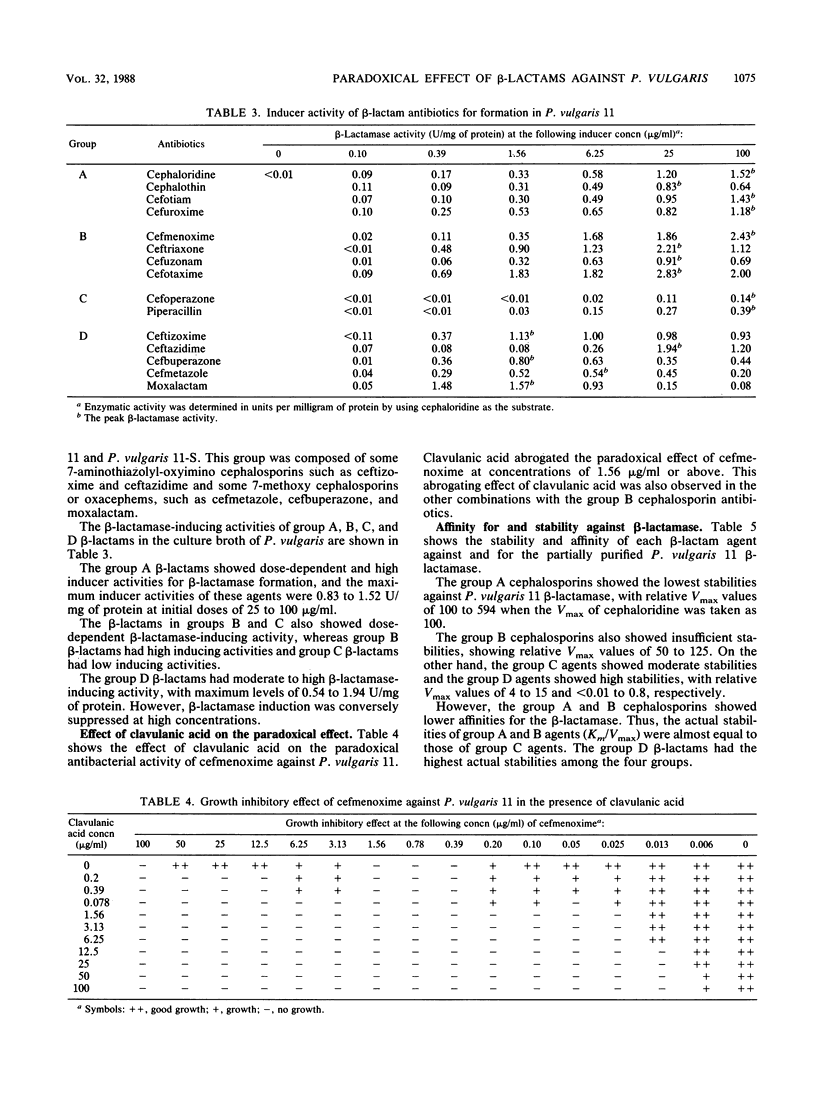
Fifteen beta-lactam antibiotics were divided into four classes based on their antibacterial actions and beta-lactamase-inducing activities in Proteus vulgaris. One of these groups, which included cefmenoxime, ceftriaxone, cefuzonam, and cefotaxime, showed a clear paradoxical antibacterial activity against P. vulgaris. This group showed growth-inhibitory activity at relatively low concentrations, up to certain limits. These cephalosporins have, as a common moiety, an aminothiazolyl-oxyimino group in the 7-acyl side chain and have high beta-lactamase-inducing activities and low stabilities against the beta-lactamase. In a mutant strain incapable of inducing beta-lactamase, however, the paradoxical antibacterial activity was not observed. These findings suggest that beta-lactamase plays an essential role in the paradoxical antibacterial effect in P. vulgaris. We conclude that the induction of a large amount of beta-lactamase and the low stability against beta-lactamase may account for the paradoxical antibacterial activity in P. vulgaris.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crumplin G. C., Smith J. T. Nalidixic acid: an antibacterial paradox. Antimicrob Agents Chemother. 1975 Sep;8(3):251–261. doi: 10.1128/aac.8.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennessey T. D., Richmond M. H. The purification and some properties of a beta-lactamase (cephalosporinase) synthesized by Enterobactercloacae. Biochem J. 1968 Sep;109(3):469–473. doi: 10.1042/bj1090469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Nishino T., Tanino T. Paradoxical antibacterial activity of cefmenoxime against Proteus vulgaris. Antimicrob Agents Chemother. 1987 Jun;31(6):865–869. doi: 10.1128/aac.31.6.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T., Nakazawa S. Bacteriological study on effects of beta-lactam group antibiotics in high concentrations. Antimicrob Agents Chemother. 1976 Jun;9(6):1033–1042. doi: 10.1128/aac.9.6.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonogi K., Kuno M., Higashide E. Induction of beta-lactamase in Proteus vulgaris. J Gen Microbiol. 1986 Jan;132(1):143–150. doi: 10.1099/00221287-132-1-143. [DOI] [PubMed] [Google Scholar]
- Okonogi K., Kuno M., Kida M., Mitsuhashi S. Beta-lactamase stability and antibacterial activity of cefmenoxime (SCE-1365), a novel cephalosporin. Antimicrob Agents Chemother. 1981 Aug;20(2):171–175. doi: 10.1128/aac.20.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reading C., Cole M. Clavulanic acid: a beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob Agents Chemother. 1977 May;11(5):852–857. doi: 10.1128/aac.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuni A. A direct spectrophotometric assay and determination of Michaelis constants for the beta-lactamase reaction. Anal Biochem. 1975 Jan;63(1):17–26. doi: 10.1016/0003-2697(75)90185-2. [DOI] [PubMed] [Google Scholar]
- Simpson I. N., Plested S. J., Harper P. B. Investigation of the beta-lactamase stability of ceftazidime and eight other new cephalosporin antibiotics. J Antimicrob Chemother. 1982 May;9(5):357–368. doi: 10.1093/jac/9.5.357. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Tokunaga M., Tokunaga H., Okajima Y., Nakae T. Characterization of porins from the outer membrane of Salmonella typhimurium. 2. Physical properties of the functional oligomeric aggregates. Eur J Biochem. 1979 Apr;95(3):441–448. doi: 10.1111/j.1432-1033.1979.tb12983.x. [DOI] [PubMed] [Google Scholar]


