Abstract
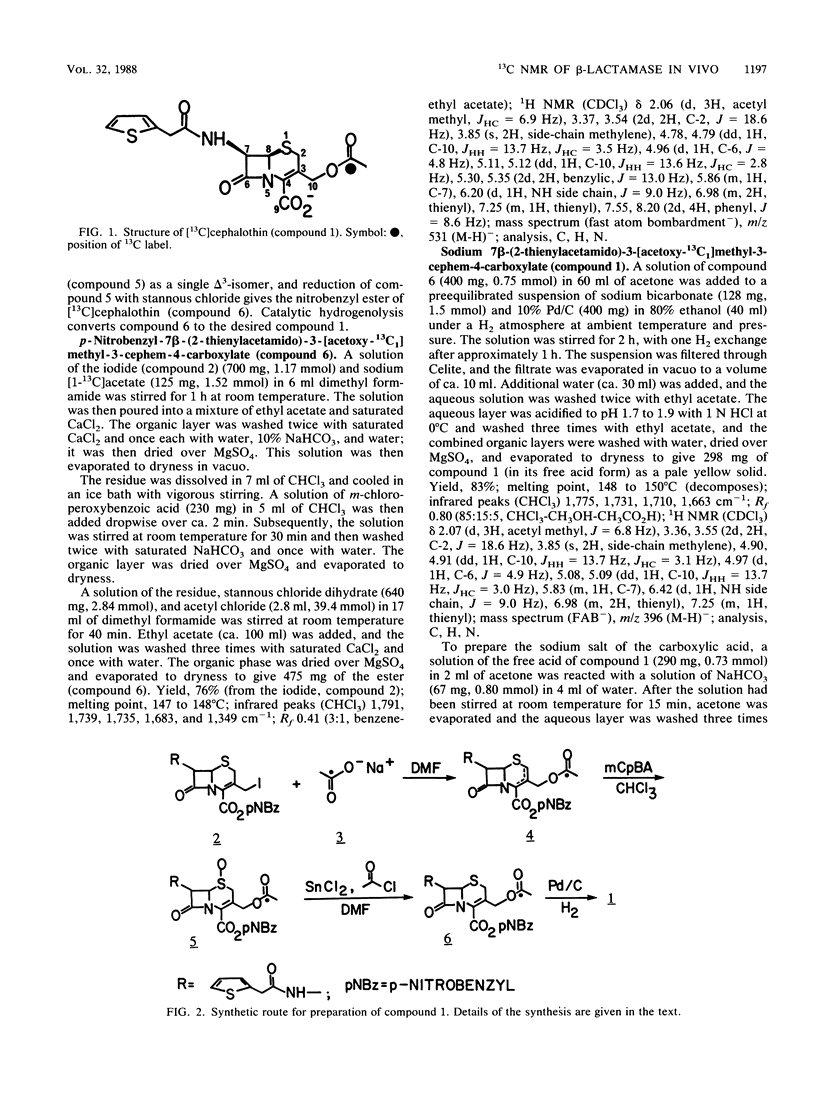
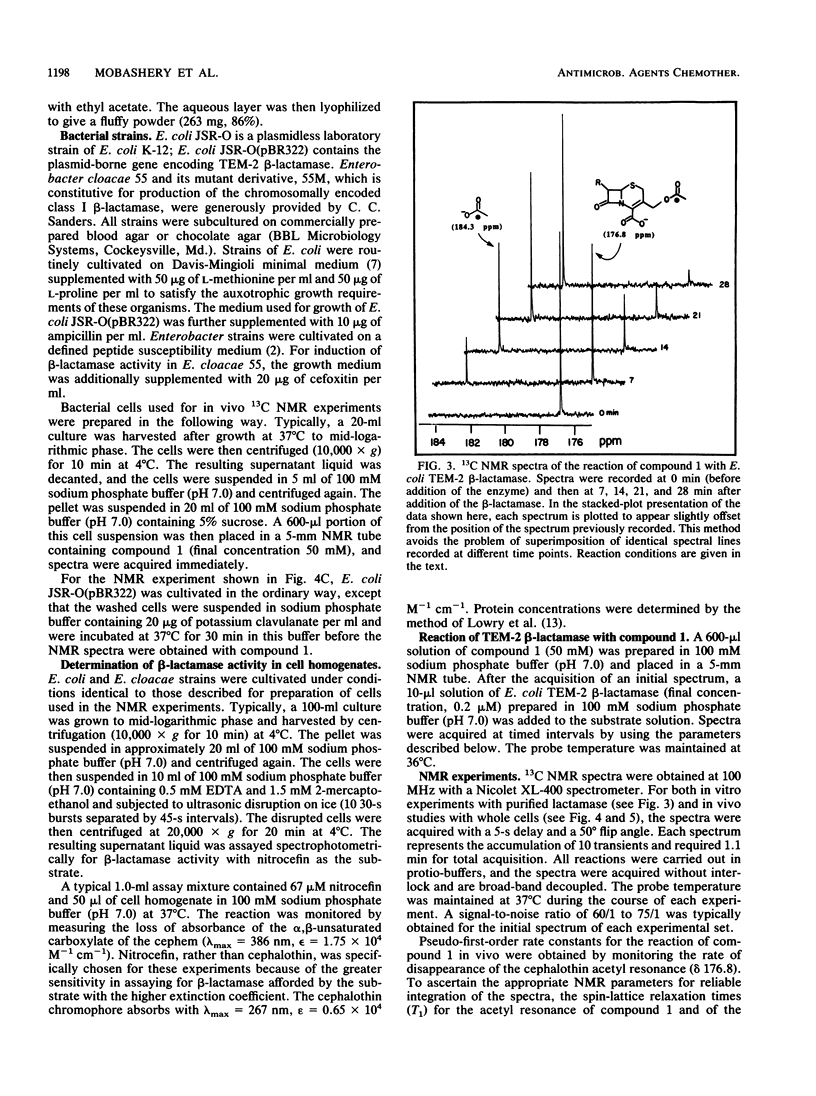
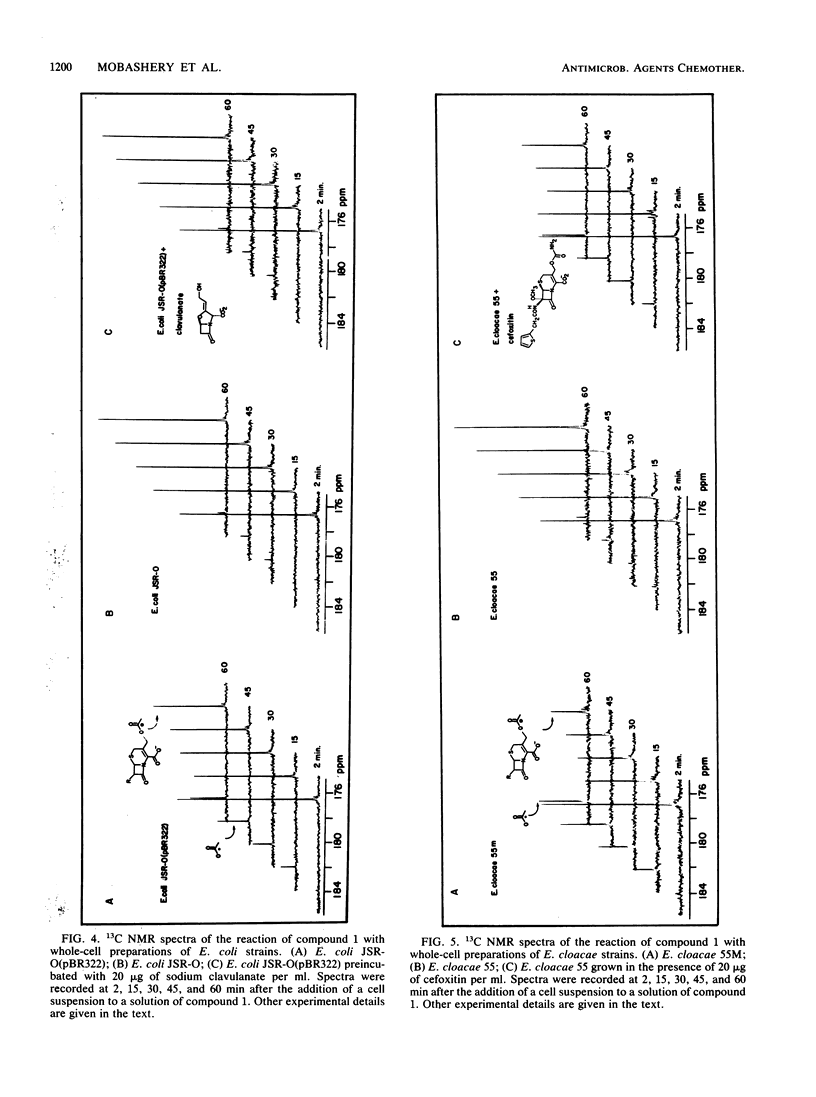
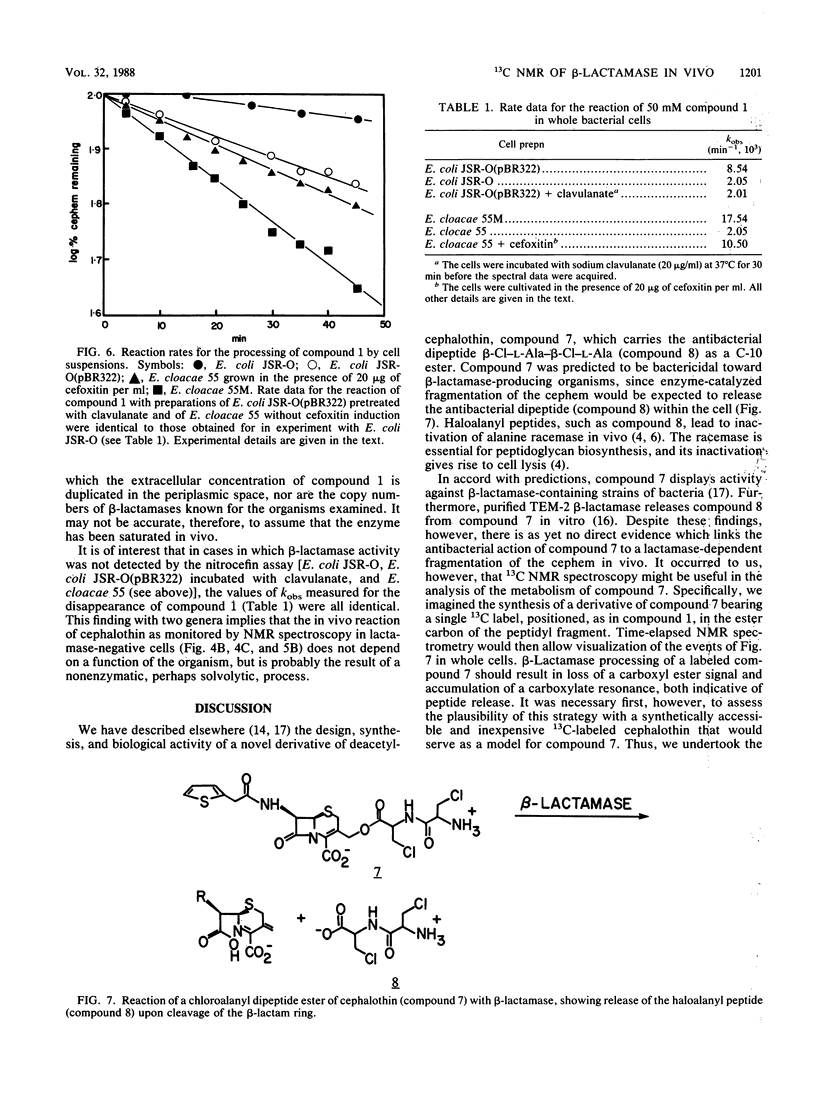
A 13C-labeled cephalothin, 7 beta-(2-thienylacetamido)-3-[acetoxy-13C1]methyl-3-cephem-4- carboxylate (compound 1), has been prepared and used to monitor beta-lactamase activities by 13C nuclear magnetic resonance spectroscopy. Time-elapsed spectral analysis of the reaction of the labeled cephalothin with the TEM-2 beta-lactamase purified from Escherichia coli revealed the progressive loss of the cephalothin acetyl resonance at 176.8 ppm and accumulation of an acetate signal at 184.3 ppm. Spectral results identical to those observed in the in vitro experiment were obtained when compound 1 was incubated with cell suspensions of E. coli JSR-O (pBR322), which contains the plasmid-encoded TEM-2 beta-lactamase, and Enterobacter cloacae strains that contain a class I chromosomal beta-lactamase. Pseudo-first-order rate constants for the lactamase-catalyzed formation of acetate from cephalothin in vivo were obtained by integration of the 13C-acetyl resonances of compound 1 during timed incubations with cell preparations. These results constitute the first demonstration of the ability to monitor beta-lactamase activity in viable cells by nuclear magnetic resonance spectroscopy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alger J. R., Sillerud L. O., Behar K. L., Gillies R. J., Shulman R. G., Gordon R. E., Shae D., Hanley P. E. In vivo carbon-13 nuclear magnetic resonance studies of mammals. Science. 1981 Nov 6;214(4521):660–662. doi: 10.1126/science.7292005. [DOI] [PubMed] [Google Scholar]
- Atherton F. R., Hall M. J., Hassall C. H., Lambert R. W., Ringrose P. S. Phosphonopeptides as antibacterial agents: rationale, chemistry, and structure-activity relationships. Antimicrob Agents Chemother. 1979 May;15(5):677–683. doi: 10.1128/aac.15.5.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boisvert W., Cheung K. S., Lerner S. A., Johnston M. Mechanisms of action of chloroalanyl antibacterial peptides. Identification of the intracellular enzymes inactivated on treatment of Escherichia coli JSR-O with the dipeptide beta Cl-LAla-beta Cl-LAla. J Biol Chem. 1986 Jun 15;261(17):7871–7878. [PubMed] [Google Scholar]
- Cheung K. S., Boisvert W., Lerner S. A., Johnston M. Chloroalanyl antibiotic peptides: antagonism of their antimicrobial effects by L-alanine and L-alanyl peptides in gram-negative bacteria. J Med Chem. 1986 Oct;29(10):2060–2068. doi: 10.1021/jm00160a045. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Vito M., Podo F., Torosantucci A., Carpinelli G., Whelan W. L., Kerridge D., Cassone A. A 19F nuclear magnetic resonance study of uptake and metabolism of 5-fluorocytosine in susceptible and resistant strains of Candida albicans. Antimicrob Agents Chemother. 1986 Feb;29(2):303–308. doi: 10.1128/aac.29.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Goering R. V. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. doi: 10.1093/infdis/146.1.34. [DOI] [PubMed] [Google Scholar]
- Kaiser G. V., Cooper R. D., Koehler R. E., Murphy C. F., Webber J. A., Wright I. G., Van Heyningen E. M. Chemistry of cephalosporin antibiotics. XIX. Transformation of delta2-cephem to delta3-cephem by oxidation-reduction at sulfur. J Org Chem. 1970 Jul;35(7):2430–2433. doi: 10.1021/jo00832a081. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mobashery S., Johnston M. Inactivation of alanine racemase by beta-chloro-L-alanine released enzymatically from amino acid and peptide C10-esters of deacetylcephalothin. Biochemistry. 1987 Sep 8;26(18):5878–5884. doi: 10.1021/bi00392a045. [DOI] [PubMed] [Google Scholar]
- Mobashery S., Johnston M. Reactions of Escherichia coli TEM beta-lactamase with cephalothin and with C10-dipeptidyl cephalosporin esters. J Biol Chem. 1986 Jun 15;261(17):7879–7887. [PubMed] [Google Scholar]
- Neurohr K. J., Barrett E. J., Shulman R. G. In vivo carbon-13 nuclear magnetic resonance studies of heart metabolism. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1603–1607. doi: 10.1073/pnas.80.6.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter H., Bickel H. Neue beta-Lactam-Antibiotika. Uber die Darstellung und Decarbonylierung von 3-Formylcephem-Verbindungen. Helv Chim Acta. 1974 Nov 6;57(7):2044–2054. doi: 10.1002/hlca.19740570715. [DOI] [PubMed] [Google Scholar]
- Shulman R. G., Brown T. R., Ugurbil K., Ogawa S., Cohen S. M., den Hollander J. A. Cellular applications of 31P and 13C nuclear magnetic resonance. Science. 1979 Jul 13;205(4402):160–166. doi: 10.1126/science.36664. [DOI] [PubMed] [Google Scholar]
- Vialaneix J. P., Chouini N., Malet-Martino M. C., Martino R., Michel G., Lepargneur J. P. Noninvasive and quantitative 19F nuclear magnetic resonance study of flucytosine metabolism in Candida strains. Antimicrob Agents Chemother. 1986 Nov;30(5):756–762. doi: 10.1128/aac.30.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman D. J., Strominger J. L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu Rev Biochem. 1983;52:825–869. doi: 10.1146/annurev.bi.52.070183.004141. [DOI] [PubMed] [Google Scholar]
- Yoshimura F., Nikaido H. Diffusion of beta-lactam antibiotics through the porin channels of Escherichia coli K-12. Antimicrob Agents Chemother. 1985 Jan;27(1):84–92. doi: 10.1128/aac.27.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]


